Molecular and functional characterization of polymorphisms in the secreted phospholipase A2 group X gene: relevance to coronary artery disease
- PMID: 19495570
- PMCID: PMC2700867
- DOI: 10.1007/s00109-009-0483-y
Molecular and functional characterization of polymorphisms in the secreted phospholipase A2 group X gene: relevance to coronary artery disease
Abstract
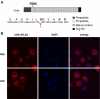
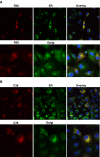
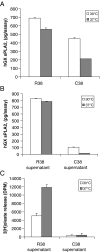
Among secreted phospholipases A2 (sPLA2s), human group X sPLA2 (hGX sPLA2) is emerging as a novel attractive therapeutic target due to its implication in inflammatory diseases. To elucidate whether hGX sPLA2 plays a causative role in coronary artery disease (CAD), we screened the human PLA2G10 gene to identify polymorphisms and possible associations with CAD end-points in a prospective study, AtheroGene. We identified eight polymorphisms, among which, one non-synonymous polymorphism R38C in the propeptide region of the sPLA2. The T-512C polymorphism located in the 5' untranslated region was associated with a decreased risk of recurrent cardiovascular events during follow-up. The functional analysis of the R38C polymorphism showed that it leads to a profound change in expression and activity of hGX sPLA2, although there was no detectable impact on CAD risk. Due to the potential role of hGX sPLA2 in inflammatory processes, these polymorphisms should be investigated in other inflammatory diseases.
Figures
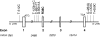

Similar articles
-
PLA2G10 Gene Variants, sPLA2 Activity, and Coronary Heart Disease Risk.Circ Cardiovasc Genet. 2015 Apr;8(2):356-62. doi: 10.1161/CIRCGENETICS.114.000633. Epub 2015 Jan 12. Circ Cardiovasc Genet. 2015. PMID: 25583995 Clinical Trial.
-
Blockade of human group X secreted phospholipase A2 (GX-sPLA2)-induced airway inflammation and hyperresponsiveness in a mouse asthma model by a selective GX-sPLA2 inhibitor.J Biol Chem. 2011 Aug 12;286(32):28049-55. doi: 10.1074/jbc.M111.235812. Epub 2011 Jun 7. J Biol Chem. 2011. PMID: 21652694 Free PMC article.
-
Key role of group v secreted phospholipase A2 in Th2 cytokine and dendritic cell-driven airway hyperresponsiveness and remodeling.PLoS One. 2013;8(2):e56172. doi: 10.1371/journal.pone.0056172. Epub 2013 Feb 25. PLoS One. 2013. PMID: 23451035 Free PMC article.
-
Function of secreted phospholipase A2 group-X in asthma and allergic disease.Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Jun;1864(6):827-837. doi: 10.1016/j.bbalip.2018.11.009. Epub 2018 Dec 5. Biochim Biophys Acta Mol Cell Biol Lipids. 2019. PMID: 30529275 Free PMC article. Review.
-
Group IID, IIE, IIF and III secreted phospholipase A2s.Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Jun;1864(6):803-818. doi: 10.1016/j.bbalip.2018.08.014. Epub 2018 Aug 31. Biochim Biophys Acta Mol Cell Biol Lipids. 2019. PMID: 30905347 Free PMC article. Review.
Cited by
-
The Roles of the Secreted Phospholipase A2 Gene Family in Immunology.Adv Immunol. 2016;132:91-134. doi: 10.1016/bs.ai.2016.05.001. Epub 2016 Jun 11. Adv Immunol. 2016. PMID: 27769509 Free PMC article. Review.
-
Secreted phospholipase A2, lipoprotein hydrolysis, and atherosclerosis: integration with lipidomics.Anal Bioanal Chem. 2011 Jun;400(7):1829-42. doi: 10.1007/s00216-011-4864-z. Epub 2011 Mar 29. Anal Bioanal Chem. 2011. PMID: 21445663 Free PMC article. Review.
-
The 763C>G Polymorphism of The Secretory PLA2IIa Gene Is Associated with Endometriosis in Iranian Women.Int J Fertil Steril. 2015 Jan-Mar;8(4):437-44. doi: 10.22074/ijfs.2015.4184. Epub 2015 Feb 7. Int J Fertil Steril. 2015. PMID: 25780526 Free PMC article.
-
A new era of secreted phospholipase A₂.J Lipid Res. 2015 Jul;56(7):1248-61. doi: 10.1194/jlr.R058123. Epub 2015 Mar 24. J Lipid Res. 2015. PMID: 25805806 Free PMC article.
-
Secretory phospholipase A2 pathway in various types of lung injury in neonates and infants: a multicentre translational study.BMC Pediatr. 2011 Nov 8;11:101. doi: 10.1186/1471-2431-11-101. BMC Pediatr. 2011. PMID: 22067747 Free PMC article. Clinical Trial.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '10491371', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10491371/'}]}
- Kugiyama K, Ota Y, Takazoe K, Moriyama Y, Kawano H, Miyao Y, Sakamoto T, Soejima H, Ogawa H, Doi H, Sugiyama S, Yasue H (1999) Circulating levels of secretory type II phospholipase A(2) predict coronary events in patients with coronary artery disease. Circulation 100:1280–1284 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1096/fj.06-6018fje', 'is_inner': False, 'url': 'https://doi.org/10.1096/fj.06-6018fje'}, {'type': 'PubMed', 'value': '17077289', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17077289/'}]}
- Karabina SA, Brocheriou I, Le Naour G, Agrapart M, Durand H, Gelb M, Lambeau G, Ninio E (2006) Atherogenic properties of LDL particles modified by human group X secreted phospholipase A2 on human endothelial cell function. FASEB J 20:2547–2549 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '17070102', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17070102/'}]}
- Rosengren B, Jonsson-Rylander AC, Peilot H, Camejo G, Hurt-Camejo E (2006) Distinctiveness of secretory phospholipase A2 group IIA and V suggesting unique roles in atherosclerosis. Biochim Biophys Acta 1761:1301–1308 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1074/jbc.M804628200', 'is_inner': False, 'url': 'https://doi.org/10.1074/jbc.m804628200'}, {'type': 'PMC', 'value': 'PMC2662271', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC2662271/'}, {'type': 'PubMed', 'value': '18801741', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/18801741/'}]}
- Sato H, Kato R, Isogai Y, Saka GI, Ohtsuki M, Taketomi Y, Yamamoto K, Tsutsumi K, Yamada J, Masuda S, Ishikawa Y, Ishii T, Kobayashi T, Ikeda K, Taguchi R, Hatakeyama S, Hara S, Kudo I, Itabe H, Murakami M (2008) Analyses of group III secreted phospholipase A2 transgenic mice reveal potential participation of this enzyme in plasma lipoprotein modification, macrophage foam cell formation, and atherosclerosis. J Biol Chem 283:33483–33497 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1161/01.ATV.0000122363.02961.c1', 'is_inner': False, 'url': 'https://doi.org/10.1161/01.atv.0000122363.02961.c1'}, {'type': 'PubMed', 'value': '14962950', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/14962950/'}]}
- Wooton-Kee CR, Boyanovsky BB, Nasser MS, de Villiers WJ, Webb NR (2004) Group V sPLA2 hydrolysis of low-density lipoprotein results in spontaneous particle aggregation and promotes macrophage foam cell formation. Arterioscler Thromb Vasc Biol 24:762–767 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

