Integration of preclinical and clinical data with pharmacokinetic modeling and simulation to evaluate fexofenadine as a probe for hepatobiliary transport function
- PMID: 19495943
- PMCID: PMC3083979
- DOI: 10.1007/s11095-009-9909-z
Integration of preclinical and clinical data with pharmacokinetic modeling and simulation to evaluate fexofenadine as a probe for hepatobiliary transport function
Abstract
Purpose: The suitability of fexofenadine as a probe substrate to assess hepatobiliary transport function in humans was evaluated by pharmacokinetic modeling/simulation and in vitro/in situ studies using chemical modulators.
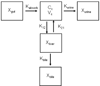
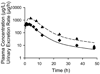
Methods: Simulations based on a pharmacokinetic model developed to describe fexofenadine disposition in humans were conducted to examine the impact of altered hepatobiliary transport on fexofenadine disposition. The effect of GF120918 on fexofenadine disposition was evaluated in human sandwich-cultured hepatocytes (SCH). Additionally, the effect of GF120918, bosentan, and taurocholate on fexofenadine disposition in perfused livers from TR(-) Wistar rats was examined.
Results: Based on modeling/simulation, fexofenadine systemic exposure was most sensitive to changes in the hepatic uptake rate constant, and did not reflect changes in hepatic exposure due to altered hepatic efflux. GF120918 did not impair fexofenadine biliary excretion in human SCH. GF120918 coadministration significantly decreased Cl'(biliary) to 27.5% of control in perfused rat livers.
Conclusions: Simulations were in agreement with perfused liver data which predicted changes in fexofenadine systemic exposure primarily due to altered hepatic uptake. Fexofenadine is not a suitable probe to assess hepatic efflux function based on systemic concentrations. GF120918-sensitive protein(s) mediate fexofenadine biliary excretion in rat liver, whereas in human hepatocytes multiple efflux proteins are involved in fexofenadine hepatobiliary disposition.
Figures


Similar articles
-
Hepatobiliary disposition of a drug/metabolite pair: Comprehensive pharmacokinetic modeling in sandwich-cultured rat hepatocytes.J Pharmacol Exp Ther. 2006 Aug;318(2):881-9. doi: 10.1124/jpet.106.102616. Epub 2006 May 11. J Pharmacol Exp Ther. 2006. PMID: 16690724
-
Multidrug resistance-associated protein 2 is primarily responsible for the biliary excretion of fexofenadine in mice.Drug Metab Dispos. 2008 Jan;36(1):61-4. doi: 10.1124/dmd.107.017319. Epub 2007 Oct 3. Drug Metab Dispos. 2008. PMID: 17913796 Free PMC article.
-
Impact of basolateral multidrug resistance-associated protein (Mrp) 3 and Mrp4 on the hepatobiliary disposition of fexofenadine in perfused mouse livers.Drug Metab Dispos. 2008 May;36(5):911-5. doi: 10.1124/dmd.107.019273. Epub 2008 Feb 14. Drug Metab Dispos. 2008. PMID: 18276836 Free PMC article.
-
Some pharmacokinetic aspects of the lipophilic terfenadine and zwitterionic fexofenadine in humans.Drugs R D. 2007;8(5):301-14. doi: 10.2165/00126839-200708050-00004. Drugs R D. 2007. PMID: 17767395 Review.
-
Fruit juice inhibition of uptake transport: a new type of food-drug interaction.Br J Clin Pharmacol. 2010 Nov;70(5):645-55. doi: 10.1111/j.1365-2125.2010.03722.x. Br J Clin Pharmacol. 2010. PMID: 21039758 Free PMC article. Review.
Cited by
-
Mechanism of efavirenz influence on methadone pharmacokinetics and pharmacodynamics.Clin Pharmacol Ther. 2012 Apr;91(4):673-84. doi: 10.1038/clpt.2011.276. Epub 2012 Mar 7. Clin Pharmacol Ther. 2012. PMID: 22398970 Free PMC article.
-
A Mechanistic Physiologically Based Pharmacokinetic (PBPK) modeling approach for fexofenadine: predictive pharmacokinetic insights in humans.Saudi Pharm J. 2025 Jul 9;33(4):24. doi: 10.1007/s44446-025-00024-4. Saudi Pharm J. 2025. PMID: 40632356 Free PMC article.
-
BDDCS, the Rule of 5 and drugability.Adv Drug Deliv Rev. 2016 Jun 1;101:89-98. doi: 10.1016/j.addr.2016.05.007. Epub 2016 May 13. Adv Drug Deliv Rev. 2016. PMID: 27182629 Free PMC article. Review.
-
Influence of seeding density and extracellular matrix on bile Acid transport and mrp4 expression in sandwich-cultured mouse hepatocytes.Mol Pharm. 2010 Apr 5;7(2):491-500. doi: 10.1021/mp900227a. Mol Pharm. 2010. PMID: 19968322 Free PMC article.
-
Optimization of Canalicular ABC Transporter Function in HuH-7 Cells by Modification of Culture Conditions.Drug Metab Dispos. 2019 Oct;47(10):1222-1230. doi: 10.1124/dmd.119.087676. Epub 2019 Aug 1. Drug Metab Dispos. 2019. PMID: 31371422 Free PMC article.
References
-
- Lippert C, Ling J, Brown P, Burmaster S, Eller M, Cheng L, Thompson R, Weir S. Mass Balance and Pharmacokinetics of MDL 16,455A in Healthy, Male Volunteers. Pharmaceutical Research. 1995;12:S-390.
-
- Tahara H, Kusuhara H, Fuse E, Sugiyama Y. P-glycoprotein plays a major role in the efflux of fexofenadine in the small intestine and blood-brain barrier, but only a limited role in its biliary excretion. Drug Metab Dispos. 2005;33:963–968. - PubMed
-
- Petri N, Tannergren C, Rungstad D, Lennernas H. Transport characteristics of fexofenadine in the Caco-2 cell model. Pharm Res. 2004;21:1398–1404. - PubMed
-
- Tannergren C, Petri N, Knutson L, Hedeland M, Bondesson U, Lennernas H. Multiple transport mechanisms involved in the intestinal absorption and first-pass extraction of fexofenadine. Clin Pharmacol Ther. 2003;74:423–436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

