Identification and characterization of heat shock 70 genes in Aedes aegypti (Diptera: Culicidae)
- PMID: 19496419
- PMCID: PMC2702248
- DOI: 10.1603/033.046.0313
Identification and characterization of heat shock 70 genes in Aedes aegypti (Diptera: Culicidae)
Abstract
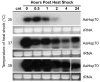
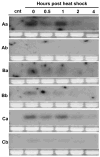
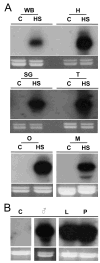
Heat shock genes are highly evolutionarily conserved and are expressed to varying degrees in all organisms in response to stress. Heat shock 70 (hsp70) genes have been well characterized in a number of organisms, most notably Drosophila melanogaster, but not as yet for any of the major arthropod-borne viral mosquito vectors. To identify hsp70 genes in the yellow fever mosquito, Aedes aegypti (Diptera: Culicidae), basic local alignment searches of the Ae. aegypti genome were performed using D. melanogaster Hsp70 protein sequences as query. Two clusters of six previously unannotated AaHsp70 genes were identified and found to be organized into three pairs of nearly identical open reading frames, which mapped to two genomic scaffolds. Consistent with a designation as heat shock genes, no detectable level of expression of AaHsp70 genes was observed under normal rearing conditions (28 degrees C), with robust expression observed with a heat shock of 37-39 degrees C. Northern analysis showed heat-inducible expression of putative AaHsp70 genes at all life stages and in all tissues tested in a time- and temperature-dependent manner. Monitoring of AaHsp70 gene expression levels in field-caught Ae. aegypti may serve as a general marker for stress. In addition, promoter sequences from AaHsp70 genes may be used to control the expression of transgenes in an inducible manner.
Figures
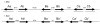


Similar articles
-
Robust heat-inducible gene expression by two endogenous hsp70-derived promoters in transgenic Aedes aegypti.Insect Mol Biol. 2012 Feb;21(1):97-106. doi: 10.1111/j.1365-2583.2011.01116.x. Epub 2011 Dec 6. Insect Mol Biol. 2012. PMID: 22142225 Free PMC article.
-
Genome-wide analysis and characterization of HSP gene families (HSP20, HSP40, HSP60, HSP70, HSP90) in the yellow fever mosquito (Aedes aegypti) (Diptera: Culicidae).J Insect Sci. 2023 Nov 1;23(6):27. doi: 10.1093/jisesa/iead114. J Insect Sci. 2023. PMID: 38102758 Free PMC article.
-
Expression of heat shock proteins (HSPs) in Aedes aegypti (L) and Aedes albopictus (Skuse) (Diptera: Culicidae) larvae in response to thermal stress.Acta Trop. 2017 Mar;167:121-127. doi: 10.1016/j.actatropica.2016.12.017. Epub 2016 Dec 24. Acta Trop. 2017. PMID: 28024869
-
Expression of AeaHsp26 and AeaHsp83 in Aedes aegypti (Diptera: Culicidae) larvae and pupae in response to heat shock stress.J Med Entomol. 2010 May;47(3):367-75. doi: 10.1603/me09232. J Med Entomol. 2010. PMID: 20496584
-
The heat shock 70 gene family in the Mediterranean fruit fly Ceratitis capitata.Insect Mol Biol. 1998 Aug;7(3):279-90. doi: 10.1111/j.1365-2583.1998.00073.x. Insect Mol Biol. 1998. PMID: 9662478
Cited by
-
Strain Variation in the Transcriptome of the Dengue Fever Vector, Aedes aegypti.G3 (Bethesda). 2012 Jan;2(1):103-14. doi: 10.1534/g3.111.001107. Epub 2012 Jan 1. G3 (Bethesda). 2012. PMID: 22384387 Free PMC article.
-
Robust heat-inducible gene expression by two endogenous hsp70-derived promoters in transgenic Aedes aegypti.Insect Mol Biol. 2012 Feb;21(1):97-106. doi: 10.1111/j.1365-2583.2011.01116.x. Epub 2011 Dec 6. Insect Mol Biol. 2012. PMID: 22142225 Free PMC article.
-
Impact of extrinsic incubation temperature on natural selection during Zika virus infection of Aedes aegypti and Aedes albopictus.PLoS Pathog. 2021 Nov 9;17(11):e1009433. doi: 10.1371/journal.ppat.1009433. eCollection 2021 Nov. PLoS Pathog. 2021. PMID: 34752502 Free PMC article.
-
Validation of a heat-inducible Ixodes scapularis HSP70 promoter and developing a tick-specific 3xP3 promoter sequence in ISE6 cells.bioRxiv [Preprint]. 2023 Nov 29:2023.11.29.569248. doi: 10.1101/2023.11.29.569248. bioRxiv. 2023. Update in: iScience. 2024 Jul 08;27(8):110468. doi: 10.1016/j.isci.2024.110468. PMID: 38076872 Free PMC article. Updated. Preprint.
-
The Temperature-Associated Effects of Rift Valley Fever Virus Infections in Mosquitoes and Climate-Driven Epidemics: A Review.Viruses. 2025 Feb 1;17(2):217. doi: 10.3390/v17020217. Viruses. 2025. PMID: 40006972 Free PMC article. Review.
References
-
- Benedict MQ, Cockburn AF, Seawright JA. The Hsp70 heat-shock gene family of the mosquito Anopheles albimanus. Insect Mol Biol. 1993;2:93–102. - PubMed
-
- Bettencourt BR, Feder ME. Hsp70 duplication in the Drosophila melanogaster species group: how and when did two become five? Molec Biol Evol. 2001;18:1272–1282. - PubMed
-
- Bettencourt BR, Feder ME. Rapid concerted evolution via gene conversion at the Drosophila hsp70 genes. J Molec Evol. 2002;54:569–586. - PubMed
-
- Craig EA. The heat shock response. CRC Crit Rev Biochem. 1985;18:239–280. - PubMed
-
- Dai H, Jiang R, Wang J, Xu G, Cao M, Wang Z, Fei J. Development of a heat shock inducible and inheritable RNAi system in silkworm. Biomolec Engl. 2007;24:625–630. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

