Comparative activities of nickel(II) and zinc(II) complexes of asymmetric [NN'O] ligands as 26S proteasome inhibitors
- PMID: 19496541
- PMCID: PMC2878368
- DOI: 10.1021/ic900276g
Comparative activities of nickel(II) and zinc(II) complexes of asymmetric [NN'O] ligands as 26S proteasome inhibitors
Abstract
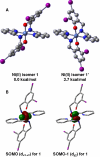
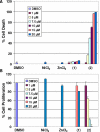
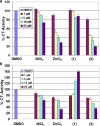
In this study, we compare the proteasome inhibition capabilities of two anticancer candidates, [Ni(L(IA))(2)] (1) and [Zn(L(IA))(2)] (2), where L(IA-) is the deprotonated form of the ligand 2,4-diiodo-6-(((2-pyridinylmethyl)amino)methyl)phenol. Species 1 contains nickel(II), a considerably inert ion that favors covalency, whereas 2 contains zinc(II), a labile transition metal ion that favors predominantly ionic bonds. We report on the synthesis and characterization of 1 and 2 using various spectroscopic, spectrometric, and structural methods. Furthermore, the pharmacological effects of 1 and 2, along with those of the salts NiCl(2) and ZnCl(2), were evaluated in vitro and in cultured human cancer cells in terms of their proteasome-inhibitory and apoptotic cell-death-inducing capabilities. It is shown that neither NiCl(2) nor 1 have the ability to inhibit the proteasome activity at any sustained levels. However, ZnCl(2) and 2 showed superior inhibitory activity versus the chymotrypsin-like activity of both the 26S proteasome (IC(50) = 5.7 and 4.4 micromol/L, respectively) and the purified 20S proteasome (IC(50) = 16.6 and 11.7 micromol/L, respectively) under cell-free conditions. Additionally, inhibition of proteasomal activity in cultured prostate cancer cells by 2 was associated with higher levels of ubiquitinated proteins and apoptosis. Treatment with either the metal complex or the salt was relatively nontoxic toward human normal cells. These results strengthen the current working hypothesis that fast ligand dissociation is required to generate an [ML(IA)](+) pharmacophore, capable of interaction with the proteasome. This interaction, possibly via N-terminal threonine amino acids present in the active sites, renders the proteasome inactive. Our results present a compelling rationale for 2 along with its gallium(III) and copper(II) congeners to be further investigated as potential anticancer drugs that act as proteasome inhibitiors.
Figures

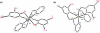



References
-
- Guo Z, Sadler PJ. Angew. Chem., Int. Ed. 1999;38:1512. - PubMed
-
- Wong E, Giandomenico CM. Chem. Rev. 1999;99:2451. - PubMed
-
- Wang D, Lippard SJ. Nat. Rev. Drug Disc. 2005;4:307. - PubMed
-
- Choi S, Vastag L, Larrabee YC, Personick ML, Schaberg KB, Fowler BJ, Sandwick RK, Rawji G. Inorg. Chem. 2008;47:1352. - PubMed
-
- Egger AE, Hartinger CG, Hamidane HB, Tsybin YO, Keppler BK, Dyson PJ. Inorg. Chem. 2008;47:10626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

