Magnetically responsive biodegradable nanoparticles enhance adenoviral gene transfer in cultured smooth muscle and endothelial cells
- PMID: 19496618
- PMCID: PMC3349935
- DOI: 10.1021/mp900017m
Magnetically responsive biodegradable nanoparticles enhance adenoviral gene transfer in cultured smooth muscle and endothelial cells
Abstract
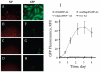
Replication-defective adenoviral (Ad) vectors have shown promise as a tool for gene delivery-based therapeutic applications. Their clinical use is however limited by therapeutically suboptimal transduction levels in cell types expressing low levels of Coxsackie-Ad receptor (CAR), the primary receptor responsible for the cell entry of the virus, and by systemic adverse reactions. Targeted delivery achievable with Ad complexed with biodegradable magnetically responsive nanoparticles (MNP) may therefore be instrumental for improving both the safety and efficiency of these vectors. Our hypothesis was that magnetically driven delivery of Ad affinity-bound to biodegradable MNP can substantially increase transgene expression in CAR deficient vascular cells in culture. Fluorescently labeled MNP were formulated from polylactide with inclusion of iron oxide and surface-modified with the D1 domain of CAR as an affinity linker. MNP cellular uptake and GFP reporter transgene expression were assayed fluorimetrically in cultured endothelial and smooth muscle cells using lambda(ex)/lambda(em) of 540 nm/575 nm and 485 nm/535 nm, respectively. Stable vector-specific association of Ad with MNP resulted in formation of MNP-Ad complexes displaying rapid cell binding kinetics following a brief exposure to a high gradient magnetic field with resultant gene transfer levels significantly increased compared to free vector or nonmagnetic control treatment. Multiple regression analysis suggested a mechanism of MNP-Ad mediated transduction distinct from that of free Ad, and confirmed the major contribution of the complexes to the gene transfer under magnetic conditions. The magnetically enhanced transduction was achieved without compromising the cell viability or growth kinetics. The enhancement of adenoviral gene delivery by affinity complexation with biodegradable MNP represents a promising approach with a potential to extend the applicability of the viral gene therapeutic strategies.
Figures




Similar articles
-
Formulation and in vitro characterization of composite biodegradable magnetic nanoparticles for magnetically guided cell delivery.Pharm Res. 2012 May;29(5):1232-41. doi: 10.1007/s11095-012-0675-y. Epub 2012 Jan 25. Pharm Res. 2012. PMID: 22274555 Free PMC article.
-
Adenoviral gene vector tethering to nanoparticle surfaces results in receptor-independent cell entry and increased transgene expression.Mol Ther. 2006 Sep;14(3):382-91. doi: 10.1016/j.ymthe.2006.03.023. Epub 2006 Jun 27. Mol Ther. 2006. PMID: 16807119
-
Site-specific gene delivery to stented arteries using magnetically guided zinc oleate-based nanoparticles loaded with adenoviral vectors.FASEB J. 2013 Jun;27(6):2198-206. doi: 10.1096/fj.12-224659. Epub 2013 Feb 13. FASEB J. 2013. PMID: 23407712 Free PMC article.
-
New generation adenoviral vectors improve gene transfer by coxsackie and adenoviral receptor-independent cell entry.Kidney Int. 2002 Jan;61(1 Suppl):S24-31. doi: 10.1046/j.1523-1755.2002.0610s1024.x. Kidney Int. 2002. PMID: 11841608 Review.
-
Targeted adenovirus vectors.Hum Gene Ther. 2004 Nov;15(11):1034-44. doi: 10.1089/hum.2004.15.1034. Hum Gene Ther. 2004. PMID: 15610604 Review.
Cited by
-
Magnetically targeted delivery of therapeutic agents to injured blood vessels for prevention of in-stent restenosis.Methodist Debakey Cardiovasc J. 2012 Jan;8(1):23-7. doi: 10.14797/mdcj-8-1-23. Methodist Debakey Cardiovasc J. 2012. PMID: 22891107 Free PMC article. Review.
-
The Progress and Promise of RNA Medicine─An Arsenal of Targeted Treatments.J Med Chem. 2022 May 26;65(10):6975-7015. doi: 10.1021/acs.jmedchem.2c00024. Epub 2022 May 9. J Med Chem. 2022. PMID: 35533054 Free PMC article. Review.
-
Computational modeling of magnetic nanoparticle targeting to stent surface under high gradient field.Comput Mech. 2014 Mar 1;53(3):403-412. doi: 10.1007/s00466-013-0968-y. Comput Mech. 2014. PMID: 24653546 Free PMC article.
-
Nanocarriers for vascular delivery of antioxidants.Nanomedicine (Lond). 2011 Sep;6(7):1257-72. doi: 10.2217/nnm.11.92. Nanomedicine (Lond). 2011. PMID: 21929460 Free PMC article. Review.
-
Microfabrication and nanotechnology in stent design.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011 May-Jun;3(3):256-68. doi: 10.1002/wnan.123. Epub 2011 Jan 31. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011. PMID: 21462356 Free PMC article. Review.
References
-
- Palmer D, Ng P. Improved system for helper-dependent adenoviral vector production. Mol. Ther. 2003;8:846–52. - PubMed
-
- Othman M, Labelle A, Mazzetti I, Elbatarny HS, Lillicrap D. Adenovirus-induced thrombocytopenia: the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood. 2007;109(7):2832–9. - PubMed
-
- Morral N, O’Neal W, Zhou H, Langston C, Beaudet A. Immune responses to reporter proteins and high viral dose limit duration of expression with adenoviral vectors: comparison of E2a wild type and E2a deleted vectors. Hum. Gene Ther. 1997;8(10):1275–86. - PubMed
-
- Tripathy SK, Black HB, Goldwasser E, Leiden JM. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat. Med. 1996;2:545–50. - PubMed
-
- Schnell MA, Zhang Y, Tazelaar J, Gao GP, Yu QC, Qian R, Chen SJ, Varnavski AN, LeClair C, Raper SE, Wilson JM. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 2001;3(5 Part 1):708–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

