Integrin function in T-cell homing to lymphoid and nonlymphoid sites: getting there and staying there
- PMID: 19496742
- PMCID: PMC2744463
- DOI: 10.1615/critrevimmunol.v29.i2.10
Integrin function in T-cell homing to lymphoid and nonlymphoid sites: getting there and staying there
Abstract
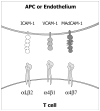
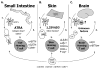
The continuous recirculation of naive T cells and their subsequent migration to tissue following activation is crucial for maintaining protective immunity against invading pathogens. The preferential targeting of effector and memory T cells to tissue is instructed during priming and mediated by cell surface expressed adhesion receptors such as integrins. Integrins arc involved in nearly all aspects of T-cell life, including naive T-cell circulation, activation, and finally effector T-cell trafficking and localization. Recent research has revealed that microenvironmental factors present during T-cell priming result in the specific regulation of adhesion/integrin and chemokine receptor expression. Once antigen-experienced T cells enter tissue, further changes in integrin expression may occur that arc critical for T-cell localization, retention, effector function, and survival. This review discusses the function of integrin expression on T cells and the multiple roles integrins play on naive T cells and in directing effector T-cell trafficking to nonlymphoid sites in order to maintain protective adaptive immunity at body barriers.
Figures

References
-
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–78. - PubMed
-
- Pribila JT, Quale AC, Mueller KL, Shimizu Y. Integrins and T cell-mediated immunity. Annu Rev Immunol. 2004;22:157–80. - PubMed
-
- Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–59. - PubMed
-
- Burbach BJ, Medeiros RB, Mueller KL, Shimizu Y. T-cell receptor signaling to integrins. Immunol Rev. 2007;218:65–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

