Zinc transporter-2 (ZnT2) variants are localized to distinct subcellular compartments and functionally transport zinc
- PMID: 19496757
- PMCID: PMC3381892
- DOI: 10.1042/BJ20081189
Zinc transporter-2 (ZnT2) variants are localized to distinct subcellular compartments and functionally transport zinc
Abstract
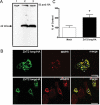
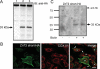
ZnT2 (zinc transporter-2) expression is restricted to tissues with unique zinc requirements such as mammary and prostate glands. We previously determined that ZnT2 plays a major role in zinc export from mammary glands, as women with a mutation in the gene encoding ZnT2 (SLC30A2) had an approximately 75% reduction in milk zinc concentration. Two distinct human ZnT2 isoforms (approximately 42 and 35 kDa) are predicted to result from alternative splicing of SLC30A2. We examined the localization and function of each ZnT2 isoform, in cells generated to express ZnT2-HA (haemagglutinin) fusion proteins. The 42 kDa isoform was localized primarily to the endosomal/secretory compartment and overexpression resulted in increased zinc vesicularization. In contrast, the 35 kDa isoform is associated with the plasma membrane. Importantly, zinc transport was higher in cells over-expressing each isoform, indicating that both proteins are functional. Endogenous expression of the secretory vesicle-associated ZnT2 isoform predominates in mammary cells and expression is higher in secreting cells, whereas the smaller isoform plays a minor role in zinc export, directly reflecting the secretory function of the mammary gland. Together our data shed further light on the complex integration of cellular zinc transport mechanisms, which may be facilitated by multiple isoforms of specific zinc transporters with unique cellular functions.
Figures





Similar articles
-
Essential Role for Zinc Transporter 2 (ZnT2)-mediated Zinc Transport in Mammary Gland Development and Function during Lactation.J Biol Chem. 2015 May 22;290(21):13064-78. doi: 10.1074/jbc.M115.637439. Epub 2015 Apr 7. J Biol Chem. 2015. PMID: 25851903 Free PMC article.
-
Exome Sequencing of SLC30A2 Identifies Novel Loss- and Gain-of-Function Variants Associated with Breast Cell Dysfunction.J Mammary Gland Biol Neoplasia. 2015 Dec;20(3-4):159-72. doi: 10.1007/s10911-015-9338-z. Epub 2015 Aug 21. J Mammary Gland Biol Neoplasia. 2015. PMID: 26293594
-
Prolactin regulates ZNT2 expression through the JAK2/STAT5 signaling pathway in mammary cells.Am J Physiol Cell Physiol. 2009 Aug;297(2):C369-77. doi: 10.1152/ajpcell.00589.2008. Epub 2009 Jun 3. Am J Physiol Cell Physiol. 2009. PMID: 19494234 Free PMC article.
-
The role of the zinc transporter SLC30A2/ZnT2 in transient neonatal zinc deficiency.Metallomics. 2017 Oct 18;9(10):1352-1366. doi: 10.1039/c7mt00162b. Metallomics. 2017. PMID: 28665435 Review.
-
The families of zinc (SLC30 and SLC39) and copper (SLC31) transporters.Curr Top Membr. 2014;73:321-55. doi: 10.1016/B978-0-12-800223-0.00009-8. Curr Top Membr. 2014. PMID: 24745988 Review.
Cited by
-
Mercury Induced Tissue Damage, Redox Metabolism, Ion Transport, Apoptosis, and Intestinal Microbiota Change in Red Swamp Crayfish (Procambarus clarkii): Application of Multi-Omics Analysis in Risk Assessment of Hg.Antioxidants (Basel). 2022 Sep 29;11(10):1944. doi: 10.3390/antiox11101944. Antioxidants (Basel). 2022. PMID: 36290667 Free PMC article.
-
Zinc and zinc transporter regulation in pancreatic islets and the potential role of zinc in islet transplantation.Rev Diabet Stud. 2010 Winter;7(4):263-74. doi: 10.1900/RDS.2010.7.263. Epub 2011 Feb 10. Rev Diabet Stud. 2010. PMID: 21713314 Free PMC article. Review.
-
Zinc transporter 8 (ZnT8) and β cell function.Trends Endocrinol Metab. 2014 Aug;25(8):415-24. doi: 10.1016/j.tem.2014.03.008. Epub 2014 Apr 18. Trends Endocrinol Metab. 2014. PMID: 24751356 Free PMC article. Review.
-
The Intestinal Transporter SLC30A1 Plays a Critical Role in Regulating Systemic Zinc Homeostasis.Adv Sci (Weinh). 2024 Dec;11(46):e2406421. doi: 10.1002/advs.202406421. Epub 2024 Oct 18. Adv Sci (Weinh). 2024. PMID: 39422023 Free PMC article.
-
ZnT2 is a critical mediator of lysosomal-mediated cell death during early mammary gland involution.Sci Rep. 2015 Jan 26;5:8033. doi: 10.1038/srep08033. Sci Rep. 2015. PMID: 25620235 Free PMC article.
References
-
- Eide DJ. The SLC39 family of metal ion transporters. Pflugers Arch. 2004;447:796–800. - PubMed
-
- Palmiter RD, Huang L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch. 2004;447:744–751. - PubMed
-
- Chowanadisai W, Lönnerdal B, Kelleher SL. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J. Biol. Chem. 2006;281:39699–39707. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

