Plasmodium berghei-infection induces volume-regulated anion channel-like activity in human hepatoma cells
- PMID: 19496788
- PMCID: PMC2774480
- DOI: 10.1111/j.1462-5822.2009.01342.x
Plasmodium berghei-infection induces volume-regulated anion channel-like activity in human hepatoma cells
Abstract
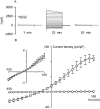
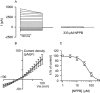
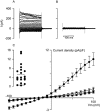
Parasite infection can lead to alterations in the permeability of host plasma membranes. Presented here is the first demonstration that this phenomenon occurs in Plasmodium-infected liver cells. Using the whole-cell patch-clamp technique, volume-regulated anion channel (VRAC) activity was characterized in Huh-7 cells (a human hepatoma cell line) before and after infection with Plasmodium berghei. Consistent with the presence of VRACs, hypotonic bath solution induced large ion currents in Huh-7 cells that rectified outwardly, reversed close to the equilibrium potential for Cl(-) and were inhibited by tamoxifen, clomiphene, mefloquine and 5-nitro-2, 3-(phenylpropylamino)-benzoic acid (NPPB), with IC(50) values of 4 +/- 1, 4 +/- 2, 2 +/- 1 and 52 +/- 12 microM respectively. In isotonic conditions, initial current recordings measured in uninfected and immature (24 h post invasion) parasite-infected Huh-7 cells were similar (with conductances of 14 +/- 3 versus 19 +/- 5 pS/pF). However, in mature (48-72 h post invasion) parasite-infected Huh-7 cells there was a sevenfold increase in currents (with a conductance of 98 +/- 16 pS/pF). The elevated currents observed in the latter are consistent with VRAC-like activity and the possible reasons for their activation are discussed.
Figures

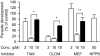
References
-
- Bodily K, Wang Y, Roman R, Sostman A, Fitz JG. Characterization of a swelling-activated anion conductance in homozygous typing cell hepatoma cells. Hepatology. 1997;25:403–410. - PubMed
-
- Boulard Y, Landau I, Miltgen F, Peters W, Ellis DS. The chemotherapy of rodent malaria XLI. Causal prophylaxis. Part V. Effect of mefloquine on exoerythrocytic schizogony in Plasmodium yoelii yoelii. Ann Trop Med Parasitol. 1986;80:577–580. - PubMed
-
- Cunha-Rodrigues M, Prudencio M, Mota MM, Haas W. Antimalarial drugs – host targets (re)visited. Biotechnol J. 2006;1:321–332. - PubMed
-
- Decherf G, Egee S, Staines HM, Ellory JC, Thomas SL. Anionic channels in malaria-infected human red blood cells. Blood Cells Mol Dis. 2004;32:366–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

