Validation of a high-throughput fermentation system based on online monitoring of biomass and fluorescence in continuously shaken microtiter plates
- PMID: 19497126
- PMCID: PMC2700080
- DOI: 10.1186/1475-2859-8-31
Validation of a high-throughput fermentation system based on online monitoring of biomass and fluorescence in continuously shaken microtiter plates
Abstract
Background: An advanced version of a recently reported high-throughput fermentation system with online measurement, called BioLector, and its validation is presented. The technology combines high-throughput screening and high-information content by applying online monitoring of scattered light and fluorescence intensities in continuously shaken microtiter plates. Various examples in calibration of the optical measurements, clone and media screening and promoter characterization are given.
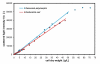
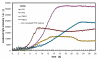
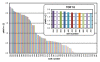
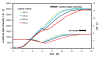
Results: Bacterial and yeast biomass concentrations of up to 50 g/L cell dry weight could be linearly correlated to scattered light intensities. In media screening, the BioLector could clearly demonstrate its potential for detecting different biomass and product yields and deducing specific growth rates for quantitatively evaluating media and nutrients. Growth inhibition due to inappropriate buffer conditions could be detected by reduced growth rates and a temporary increase in NADH fluorescence. GFP served very well as reporter protein for investigating the promoter regulation under different carbon sources in yeast strains. A clone screening of 90 different GFP-expressing Hansenula polymorpha clones depicted the broad distribution of growth behavior and an even stronger distribution in GFP expression. The importance of mass transfer conditions could be demonstrated by varying filling volumes of an E. coli culture in 96 well MTP. The different filling volumes cause a deviation in the culture growth and acidification both monitored via scattered light intensities and the fluorescence of a pH indicator, respectively.
Conclusion: The BioLector technology is a very useful tool to perform quantitative microfermentations under engineered reaction conditions. With this technique, specific yields and rates can be directly deduced from online biomass and product concentrations, which is superior to existing technologies such as microplate readers or optode-based cultivation systems. In particular, applications with strong demand on high-throughput such as clone and media screening and systems biology can benefit from its simple handling, the high quantitative information content and its capacity of automation.
Figures






References
-
- Maharbiz MM, Holtz WJ, Howe RT, Keasling JD. Microbioreactor arrays with parametric control for high-throughput experimentation. Biotechnol Bioeng. 2004;86:485–490. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

