Production of self-assembling biomaterials for tissue engineering
- PMID: 19497631
- PMCID: PMC2828541
- DOI: 10.1016/j.tibtech.2009.04.002
Production of self-assembling biomaterials for tissue engineering
Abstract
Self-assembling peptide-based biomaterials are being developed for use as 3D tissue engineering scaffolds and for therapeutic drug-release applications. Chemical synthesis provides custom-made peptides in small quantities, but production approaches based upon transgenic organisms might be more cost-effective for large-scale peptide production. Long lead times for developing appropriate animal clones or plant lines and potential negative public opinion are obstacles to these routes. Microbes, particularly safe organisms used in the food industry, offer a more rapid route to the large-scale production of recombinant self-assembling biomaterials. In this review, recent advances and challenges in the recombinant production of collagen, elastin and de novo designed self-assembling peptides are discussed.
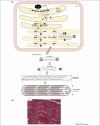
Figures



References
-
- Zhang S. Emerging biological materials through molecular self-assembly. Biotechnol. Adv. 2002;20:321–339. - PubMed
-
- Mitraki A., van Raaij M.J. Folding of β-structured fibrous proteins and self-assembling peptides. Methods Mol. Biol. 2005;300:125–140. - PubMed
-
- Kluge J.A. Spider silks and their applications. Trends Biotechnol. 2008;26:244–251. - PubMed
-
- Shapiro F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur. Cell. Mater. 2008;15:53–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

