Optimal neuroprotection by erythropoietin requires elevated expression of its receptor in neurons
- PMID: 19497871
- PMCID: PMC2701030
- DOI: 10.1073/pnas.0901840106
Optimal neuroprotection by erythropoietin requires elevated expression of its receptor in neurons
Abstract
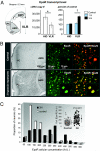
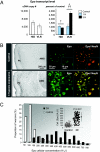
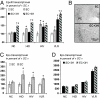
Erythropoietin receptor (EpoR) binding mediates neuroprotection by endogenous Epo or by exogenous recombinant human (rh)Epo. The level of EpoR gene expression may determine tissue responsiveness to Epo. Thus, harnessing the neuroprotective power of Epo requires an understanding of the Epo-EpoR system and its regulation. We tested the hypothesis that neuronal expression of EpoR is required to achieve optimal neuroprotection by Epo. The ventral limbic region (VLR) in the rat brain was used because we determined that its neurons express minimal EpoR under basal conditions, and they are highly sensitive to excitotoxic damage, such as occurs with pilocarpine-induced status epilepticus (Pilo-SE). We report that (i) EpoR expression is significantly elevated in nearly all VLR neurons when rats are subjected to 3 moderate hypoxic exposures, with each separated by a 4-day interval; (ii) synergistic induction of EpoR expression is achieved in the dorsal hippocampus and neocortex by the combination of hypoxia and exposure to an enriched environment, with minimal increased expression by either treatment alone; and (iii) rhEpo administered after Pilo-SE cannot rescue neurons in the VLR, unless neuronal induction of EpoR is elicited by hypoxia before Pilo-SE. This study thus demonstrates using environmental manipulations in normal rodents, the strict requirement for induction of EpoR expression in brain neurons to achieve optimal neuroprotection. Our results indicate that regulation of EpoR gene expression may facilitate the neuroprotective potential of rhEpo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

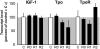
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

