Adrenergic activation of electrogenic K+ secretion in guinea pig distal colonic epithelium: desensitization via the Y2-neuropeptide receptor
- PMID: 19497958
- PMCID: PMC2724082
- DOI: 10.1152/ajpgi.00077.2009
Adrenergic activation of electrogenic K+ secretion in guinea pig distal colonic epithelium: desensitization via the Y2-neuropeptide receptor
Abstract
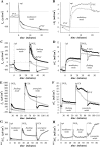
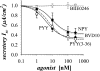
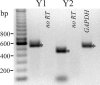
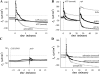
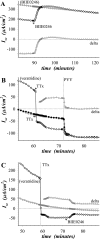
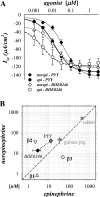
Adrenergic activation of electrogenic K+ secretion in isolated mucosa from guinea pig distal colon was desensitized by peptide-YY (PYY). Addition of PYY or neuropeptide-Y (NPY) to the bathing solution of mucosae in Ussing chambers suppressed the short-circuit current (Isc) corresponding to electrogenic Cl- secretion, whether stimulated by epinephrine (epi), prostaglandin-E2 (PGE2), or carbachol (CCh). Neither peptide markedly inhibited the large transient component of synergistic secretion (PGE2 + CCh). Sustained Cl- secretory Isc was inhibited approximately 65% by PYY or NPY, with IC50s of 4.1 +/- 0.9 nM and 9.4 +/- 3.8 nM, respectively. This inhibition was eliminated by BIIE0246, an antagonist of the Y2-neuropeptide receptor (Y2-NpR), but not by Y1-NpR antagonist BVD10. Adrenergic sensitivity for activation of K+ secretion in the presence of Y2-NpR blockade by BIIE0246 was (EC50s) 2.9 +/- 1.2 nM for epi and 13.3 +/- 1.0 nM for norepinephrine, approximately fourfold greater than in the presence of PYY. Expression of mRNA for both Y1-NpR and Y2-NpR was indicated by RT-PCR of RNA from colonic mucosa, and protein expression was indicated by immunoblot. Immunoreactivity (ir) for Y1-NpR and Y2-NpR was distinct in basolateral membranes of columnar epithelial cells in the crypts of Lieberkühn as well as intercrypt surface epithelium. Adrenergic nerves in proximity with crypts were detected by ir for dopamine-beta-hydroxylase, and a portion of these nerves also contained NPY(ir). BIIE0246 addition increased secretagog-activated Isc, consistent with in vitro release of either PYY or NPY. Thus PYY and NPY were able to suppress Cl- secretory capacity and desensitize the adrenergic K+ secretory response, providing a direct inhibitory counterbalance against secretory activation.
Figures






Similar articles
-
beta-Adrenergic activation of electrogenic K+ and Cl- secretion in guinea pig distal colonic epithelium proceeds via separate cAMP signaling pathways.Am J Physiol Gastrointest Liver Physiol. 2010 Jul;299(1):G81-95. doi: 10.1152/ajpgi.00035.2010. Epub 2010 Apr 22. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20413718 Free PMC article.
-
Adrenergic activation of electrogenic K+ secretion in guinea pig distal colonic epithelium: involvement of beta1- and beta2-adrenergic receptors.Am J Physiol Gastrointest Liver Physiol. 2009 Aug;297(2):G269-77. doi: 10.1152/ajpgi.00076.2009. Epub 2009 May 21. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19460844 Free PMC article.
-
Functional consequences of neuropeptide Y Y 2 receptor knockout and Y2 antagonism in mouse and human colonic tissues.Br J Pharmacol. 2003 Jun;139(4):863-71. doi: 10.1038/sj.bjp.0705298. Br J Pharmacol. 2003. PMID: 12813010 Free PMC article.
-
Neuropeptide Y receptor subtypes, Y1 and Y2.Ann N Y Acad Sci. 1990;611:7-26. doi: 10.1111/j.1749-6632.1990.tb48918.x. Ann N Y Acad Sci. 1990. PMID: 2174225 Review.
-
Neuropeptide Y Y1 receptor mechanisms in sympathetic vascular control.Acta Physiol Scand Suppl. 1997;636:1-55. Acta Physiol Scand Suppl. 1997. PMID: 9179320 Review.
Cited by
-
Colonic potassium handling.Pflugers Arch. 2010 Apr;459(5):645-56. doi: 10.1007/s00424-009-0781-9. Epub 2010 Feb 10. Pflugers Arch. 2010. PMID: 20143237 Review.
-
Activation of the basolateral membrane Cl- conductance essential for electrogenic K+ secretion suppresses electrogenic Cl- secretion.Exp Physiol. 2011 Mar;96(3):305-16. doi: 10.1113/expphysiol.2010.055038. Epub 2010 Dec 17. Exp Physiol. 2011. PMID: 21169331 Free PMC article.
-
beta-Adrenergic activation of electrogenic K+ and Cl- secretion in guinea pig distal colonic epithelium proceeds via separate cAMP signaling pathways.Am J Physiol Gastrointest Liver Physiol. 2010 Jul;299(1):G81-95. doi: 10.1152/ajpgi.00035.2010. Epub 2010 Apr 22. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20413718 Free PMC article.
-
Peripheral peptide YY inhibits propulsive colonic motor function through Y2 receptor in conscious mice.Am J Physiol Gastrointest Liver Physiol. 2010 Jan;298(1):G45-56. doi: 10.1152/ajpgi.00349.2009. Epub 2009 Nov 5. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 19892938 Free PMC article.
-
Role of the BK channel (KCa1.1) during activation of electrogenic K+ secretion in guinea pig distal colon.Am J Physiol Gastrointest Liver Physiol. 2012 Dec 15;303(12):G1322-34. doi: 10.1152/ajpgi.00325.2012. Epub 2012 Oct 11. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 23064759 Free PMC article.
References
-
- Anderson GP Current issues with β2-adrenoceptor agonists: pharmacology and molecular and cellular mechanisms. Clin Rev Allergy Immunol 31: 119–130, 2006. - PubMed
-
- Ballantyne GH, Goldenring JR, Savoca PE, Kranz HK, Adrian TE, Bilchik AJ, Modlin IM. Cyclic AMP-mediated release of peptide YY (PYY) from the isolated perfused rabbit distal colon. Regul Pept 47: 117–126, 1993. - PubMed
-
- Barrett KE, Huott PA, Shah SS, Dharmsathaphorn K, Wasserman SI. Differing effects of apical and basolateral adenosine on colonic epithelial cell line T84. Am J Physiol Cell Physiol 256: C197–C203, 1989. - PubMed
-
- Berglund MM, Hipskind PA, Gehlert DR. Function, Distribution and Molecular Pharmacology of Npy-Family Receptors: The Npy Family of Peptides in Immune Disorders, Inflammation, Angiogenesis and Cancer, edited by Zukowska Z, Feuerstein GZ. Boston: Birkhäuser Verlag, 2005, p. 29–62.
-
- Brechet S, Plaisancié P, Dumoulin V, Chayvialle JA, Cuber JC, Claustre J. Involvement of β1- and β2- but not β3-adrenoceptor activation in adrenergic PYY secretion from the isolated colon. J Endocrinol 168: 177–183, 2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

