The mediator complex subunit 1 enhances transcription of genes needed for adrenal androgen production
- PMID: 19497978
- PMCID: PMC2736083
- DOI: 10.1210/en.2009-0006
The mediator complex subunit 1 enhances transcription of genes needed for adrenal androgen production
Abstract
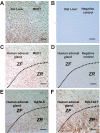
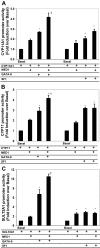
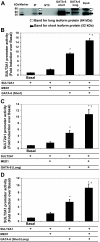
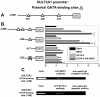
There are three enzymes involved in the biosynthesis of the adrenal androgen dehydroepiandrosterone (DHEA) sulfate. Cholesterol side-chain cleavage (CYP11A1) and 17alpha-hydroxylase/17,20-lyase (CYP17) metabolize cholesterol into DHEA, whereas steroid sulfotransferase family 2A1 (SULT2A1) is responsible for conversion of DHEA to DHEA sulfate. We previously examined the mechanisms regulating CYP11A1, CYP17, and SULT2A1 transcription and found that each is regulated, in part, by the transcription factor GATA-6. Previous studies suggested that mediator complex subunit 1 (MED1, also called PPARBP or TRAP220) is a cofactor involved in not only the regulation of nuclear receptors but also the activation of GATA-6 transcription. Herein we demonstrated a role for MED1 in the regulation of CYP11A1, CYP17, and SULT2A1 transcription. Transient transfection assays with SULT2A1 deletion and mutation promoter constructs allowed the determination of specific the GATA-6 binding cis-regulatory elements necessary for transactivation of SULT2A1 transcription. Binding of MED1 and GATA-6 was confirmed by coimmunoprecipitation/Western analysis and chromatin immunoprecipitation assay. We demonstrated expression of MED1 mRNA and protein in the human adrenal and determined that knockdown of MED1 expression via specific small interfering RNA attenuated CYP11A1, CYP17, and SULT2A1 expression levels in H295R cells. In addition, we demonstrated that MED1 enhanced GATA-6 stimulated transcription of promoter constructs for each of these genes. Moreover, the activity of MED1 for SULT2A1 promoter was mediated by GATA-6 via the -190 GATA-binding site. These data support the hypothesis that MED1 and GATA-6 are key regulators of SULT2A1 expression, and they play important roles in adrenal androgen production.
Figures


Similar articles
-
GATA-6 is expressed in the human adrenal and regulates transcription of genes required for adrenal androgen biosynthesis.Endocrinology. 2003 Oct;144(10):4285-8. doi: 10.1210/en.2003-0472. Epub 2003 Jul 31. Endocrinology. 2003. PMID: 12959982
-
Steroid sulfotransferase 2A1 gene transcription is regulated by steroidogenic factor 1 and GATA-6 in the human adrenal.Mol Endocrinol. 2005 Jan;19(1):184-97. doi: 10.1210/me.2003-0332. Epub 2004 Sep 23. Mol Endocrinol. 2005. PMID: 15388788
-
Regulation of the adrenal androgen biosynthesis.J Steroid Biochem Mol Biol. 2008 Feb;108(3-5):281-6. doi: 10.1016/j.jsbmb.2007.09.015. Epub 2007 Sep 11. J Steroid Biochem Mol Biol. 2008. PMID: 17945481 Free PMC article. Review.
-
Transcriptional regulation of dehydroepiandrosterone sulfotransferase (SULT2A1) by estrogen-related receptor alpha.Endocrinology. 2005 Aug;146(8):3605-13. doi: 10.1210/en.2004-1619. Epub 2005 May 5. Endocrinology. 2005. PMID: 15878968
-
Androgen synthesis in adrenarche.Rev Endocr Metab Disord. 2009 Mar;10(1):3-17. doi: 10.1007/s11154-008-9102-4. Rev Endocr Metab Disord. 2009. PMID: 18821018 Review.
Cited by
-
Combined deletion of Fxr and Shp in mice induces Cyp17a1 and results in juvenile onset cholestasis.J Clin Invest. 2011 Jan;121(1):86-95. doi: 10.1172/JCI42846. Epub 2010 Dec 1. J Clin Invest. 2011. PMID: 21123943 Free PMC article.
-
Drosophila Mediator Subunit Med1 Is Required for GATA-Dependent Developmental Processes: Divergent Binding Interfaces for Conserved Coactivator Functions.Mol Cell Biol. 2019 Mar 19;39(7):e00477-18. doi: 10.1128/MCB.00477-18. Print 2019 Apr 1. Mol Cell Biol. 2019. PMID: 30670567 Free PMC article.
-
Human adrenal cells that express both 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2) and cytochrome b5 (CYB5A) contribute to adrenal androstenedione production.J Steroid Biochem Mol Biol. 2011 Feb;123(3-5):122-6. doi: 10.1016/j.jsbmb.2010.12.001. Epub 2010 Dec 23. J Steroid Biochem Mol Biol. 2011. PMID: 21185375 Free PMC article.
-
Toying with fate: Redirecting the differentiation of adrenocortical progenitor cells into gonadal-like tissue.Mol Cell Endocrinol. 2015 Jun 15;408:165-77. doi: 10.1016/j.mce.2014.12.003. Epub 2014 Dec 8. Mol Cell Endocrinol. 2015. PMID: 25498963 Free PMC article. Review.
-
Integrating LCM-Based Spatio-Temporal Transcriptomics Uncovers Conceptus and Endometrial Luminal Epithelium Communication that Coordinates the Conceptus Attachment in Pigs.Int J Mol Sci. 2021 Jan 27;22(3):1248. doi: 10.3390/ijms22031248. Int J Mol Sci. 2021. PMID: 33513863 Free PMC article.
References
-
- Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI 2002 Dissecting human adrenal androgen production. Trends Endocrinol Metab 13:234–239 - PubMed
-
- Kennerson AR, McDonald DA, Adams JB 1983 Dehydroepiandrosterone sulfotransferase localization in human adrenal glands: a light and electron microscopic study. J Clin Endocrinol Metab 56:786–790 - PubMed
-
- Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, Rainey WE 2000 Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf) 53:739–747 - PubMed
-
- Saner KJ, Suzuki T, Sasano H, Pizzey J, Ho C, Strauss JF, Carr BR, Rainey WE 2005 Steroid sulfotransferase 2A1 gene transcription is regulated by steroidogenic factor 1 and GATA-6 in the human adrenal. Mol Endocrinol 19:184–197 - PubMed
-
- Seely J, Amigh KS, Suzuki T, Mayhew B, Sasano H, Giguere V, Laganière J, Carr BR, Rainey WE 2005 Transcriptional regulation of dehydroepiandrosterone sulfotransferase (SULT2A1) by estrogen-related receptor α. Endocrinology 146:3605–3613 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

