The bone morphogenetic protein signaling pathway is upregulated in a mouse model of total parenteral nutrition
- PMID: 19498022
- PMCID: PMC2696986
- DOI: 10.3945/jn.108.096669
The bone morphogenetic protein signaling pathway is upregulated in a mouse model of total parenteral nutrition
Abstract
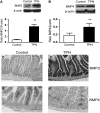
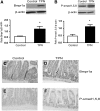
Total parenteral nutrition (TPN) results in intestinal mucosal atrophic changes due to an absence of enteral nutrition; however, the mechanisms responsible for this are not fully understood. It has been shown that bone morphogenetic protein (BMP) activation inhibits intestinal epithelial cell (EC) proliferation. Therefore, we hypothesized that the BMP pathway could be upregulated by TPN. To address this, we randomly assigned mice to receive TPN or to be enterally fed (control) for 7 d. Mucosal EC isolates were harvested from the entire length of small intestine for RNA and protein measurements. Full-thickness, mid-small bowel was processed for histological examination. TPN increased the abundance of BMP2, BMP4, and BMP type II receptor at the RNA and protein levels. Phosphorylation of Smad1, Smad5, and Smad8 also was greater in the TPN group than in the control, which helped to confirm activation of this pathway. Interestingly, the TPN and control groups did not differ in the mRNA expression of the extracellular soluble bmp antagonists, noggin, gremlin, chordin, or follistatin. Compared to the control group, the expression of c-Myc (cellular myelocytomatosis) mRNA was lower, whereas the level of p21(WAF1/CIP1) was greater, in the TPN group. Because the BMP family may function through suppression of Wnt-beta-catenin signaling, this pathway was also examined. mRNA expression of Wnt 3, Wnt5a, and the Wnt receptor Lrp5 were lower in the TPN group compared to controls. The results suggest that the BMP signaling pathway may be involved in the development of intestinal mucosal atrophy due to TPN administration.
Figures


References
-
- Yang H, Antony PA, Wildhaber BE, Teitelbaum DH. Intestinal intraepithelial lymphocyte gammadelta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol. 2004;172:4151–8. - PubMed
-
- Wildhaber BE, Yang H, Spencer AU, Drongowski RA, Teitelbaum DH. Lack of enteral nutrition–effects on the intestinal immune system. J Surg Res. 2005;123:8–16. - PubMed
-
- Massagué J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–91. - PubMed
-
- He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–21. - PubMed
-
- Haramis AP, Begthel H, van den Born M, van Es J, Jonkheer S, Offerhaus G, Clevers H. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

