Nanocarriers' entry into the cell: relevance to drug delivery
- PMID: 19499185
- PMCID: PMC11115599
- DOI: 10.1007/s00018-009-0053-z
Nanocarriers' entry into the cell: relevance to drug delivery
Abstract
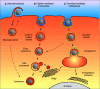
Nanocarriers offer unique possibilities to overcome cellular barriers in order to improve the delivery of various drugs and drug candidates, including the promising therapeutic biomacromolecules (i.e., nucleic acids, proteins). There are various mechanisms of nanocarrier cell internalization that are dramatically influenced by nanoparticles' physicochemical properties. Depending on the cellular uptake and intracellular trafficking, different pharmacological applications may be considered. This review will discuss these opportunities, starting with the phagocytosis pathway, which, being increasingly well characterized and understood, has allowed several successes in the treatment of certain cancers and infectious diseases. On the other hand, the non-phagocytic pathways encompass various complicated mechanisms, such as clathrin-mediated endocytosis, caveolae-mediated endocytosis and macropinocytosis, which are more challenging to control for pharmaceutical drug delivery applications. Nevertheless, various strategies are being actively investigated in order to tailor nanocarriers able to deliver anticancer agents, nucleic acids, proteins and peptides for therapeutic applications by these non-phagocytic routes.
Figures





References
-
- Black C, Gregoriadis G. Intracellular fate and effect of liposome-entrapped actinomycin-d injected into rats. Biochem Soc Trans. 1974;2:869–871.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

