The drug release study of ceftriaxone from porous hydroxyapatite scaffolds
- PMID: 19499343
- PMCID: PMC2802160
- DOI: 10.1208/s12249-009-9265-7
The drug release study of ceftriaxone from porous hydroxyapatite scaffolds
Abstract
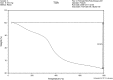
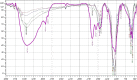
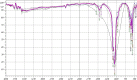
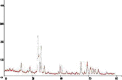
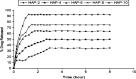
Hydroxyapatite (HAP) is an important biomedical material that is used for grafting osseous defects. It has an excellent bioactivity and biocompatibility properties. To isolate hydroxyapatite, pieces of cleaned cattle's bone were heated at different temperature range from 400 degrees C up to 1,200 degrees C. A reasonable yield of 60.32% w/w HAP was obtained at temperature range from 1,000 degrees C to 1,200 degrees C. Fourier transform infrared spectra and the thermogravimetric measurement showed a clear removal of organic at 600 degrees C as well as an excellent isolation of HAP from the bones which was achieved at 1,000-1,200 degrees C. This was also confirmed from X-ray diffraction of bone sample heated at 1,200 degrees C. The concentration ions were found to be sodium, potassium, lithium, zinc, copper, iron, calcium, magnesium, and phosphate present in bones within the acceptable limits for its role in the bioactivity property of HAP. Glucose powder was used as a porosifier. Glucose was novel and excellent as porogen where it was completely removed by heating, giving an efficient porosity in the used scaffolds. The results exhibited that the ceftriaxone drug release was increased with increasing the porosity. It was found that a faster, higher, and more regular drug release was obtained from the scaffold with a porosity of 10%.
Figures
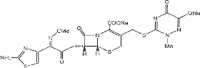
References
-
- Shalaby SW, Salz U. Polymers for dental and orthopedics applications. Boca Raton: CRC; 2007. pp. 79–80.
-
- Chakraborty S, Bag S, Pal S, Mukherjee AK. Structural and microstructural characterization of bioapatite using X-ray powder diffraction and Fourier transform infrared technique. J Appl Cryst. 2006;39:385–90. doi: 10.1107/S0021889806010351. - DOI
-
- Mucalo MR, Foster DL, Wielage B, Steinhaeuser S, Mucha H, Knichton D, Kirby J. Appl Biomat Biomech. 2004;2:96–104. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

