Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays
- PMID: 19499548
- PMCID: PMC2747737
- DOI: 10.1002/ijc.24509
Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays
Abstract
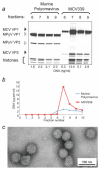
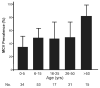
Merkel cell polyomavirus (MCV) is a newly-discovered human tumor virus found in approximately 80% of Merkel cell carcinoma (MCC). The rate of MCV infection among persons without MCC is unknown. We developed a MCV virus-like particle (VLP) enzyme-linked immunoassay (EIA) that does not cross-react with human BK or murine polyomaviruses. Peptide mapping of the MCV VP1 gene and immunoblotting with denatured MCV VLP are less sensitive than the MCV EIA in detecting MCV antibodies suggesting antibody reactivity in this assay primarily targets conformational but not linear epitopes. Among MCC patients, all 21 (100%) patients tested with MCV-positive tumors had high serum MCV IgG but not high MCV IgM levels. Only 3 of 6 (50%) MCC patients with MCV-negative tumors were positive for MCV antibodies. Sera from most adults, including 107 of 166 (64%) blood donors, 63 of 100 (63%) commercial donors and 37 of 50 (74%) systemic lupus erythematosus patients, show evidence for prior MCV exposure. Age-specific MCV prevalence was determined by examining a cross-sectional distribution of 150 Langerhans cell histiocytosis (an unrelated neoplasm) patient sera. MCV prevalence increases from 50% among children age 15 years or younger to 80% among persons older than 50 years. We did not find evidence for vertical transmission among infants. Although past exposure to MCV is common among all adult groups, MCC patients have a markedly elevated MCV IgG response compared with control patients. Our study demonstrates that MCV is a widespread but previously unrecognized human infection.
2009 UICC
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

