Slc11a1 limits intracellular growth of Salmonella enterica sv. Typhimurium by promoting macrophage immune effector functions and impairing bacterial iron acquisition
- PMID: 19500110
- PMCID: PMC3467104
- DOI: 10.1111/j.1462-5822.2009.01337.x
Slc11a1 limits intracellular growth of Salmonella enterica sv. Typhimurium by promoting macrophage immune effector functions and impairing bacterial iron acquisition
Abstract
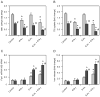
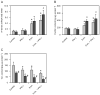
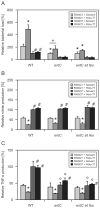
The natural resistance-associated macrophage protein 1, Slc11a1, is a phagolysosomal transporter for protons and divalent ions including iron that confers host protection against diverse intracellular pathogens including Salmonella. We investigated and compared the regulation of iron homeostasis and immune function in RAW264.7 murine phagocytes stably transfected with non-functional Slc11a1 and functional Slc11a1 controls in response to an infection with Salmonella enterica serovar Typhimurium. We report that macrophages lacking functional Slc11a1 displayed an increased expression of transferrin receptor 1, resulting in enhanced acquisition of transferrin-bound iron. In contrast, cellular iron release mediated via ferroportin 1 was significantly lower in Salmonella-infected Slc11a1-negative macrophages in comparison with phagocytes bearing Slc11a1. Lack of Slc11a1 led to intracellular persistence of S. enterica serovar Typhimurium within macrophages, which was paralleled by a reduced formation of nitric oxide, tumour necrosis factor-alpha and interleukin-6 in Slc11a1-negative macrophages following Salmonella infection, whereas interleukin-10 production was increased. Moreover, Slc11a1-negative phagocytes exhibited higher cellular iron content, resulting in increased iron acquisition by intracellular Salmonella. Our observations indicate a bifunctional role for Slc11a1 within phagocytes. Slc11a restricts iron availability, which first augments pro-inflammatory macrophage effector functions and second concomitantly limits microbial iron access.
Figures





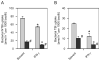
References
-
- Ables GP, Takamatsu D, Noma H, El-Shazly S, Jin HK, Taniguchi T, et al. The roles of Nramp1 and Tnfa genes in nitric oxide production and their effect on the growth of Salmonella typhimurium in macrophages from Nramp1 congenic and tumor necrosis factor-alpha−/− mice. J Interferon Cytokine Res. 2001;21:53–62. - PubMed
-
- Barton CH, Biggs TE, Baker ST, Bowen H, Atkinson PG. Nramp1: a link between intracellular iron transport and innate resistance to intracellular pathogens. J Leukoc Biol. 1999;66:757–762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

