Human T cell recognition of the blood stage antigen Plasmodium hypoxanthine guanine xanthine phosphoribosyl transferase (HGXPRT) in acute malaria
- PMID: 19500406
- PMCID: PMC2700129
- DOI: 10.1186/1475-2875-8-122
Human T cell recognition of the blood stage antigen Plasmodium hypoxanthine guanine xanthine phosphoribosyl transferase (HGXPRT) in acute malaria
Abstract
Background: The Plasmodium purine salvage enzyme, hypoxanthine guanine xanthine phosphoribosyl transferase (HGXPRT) can protect mice against Plasmodium yoelii pRBC challenge in a T cell-dependent manner and has, therefore, been proposed as a novel vaccine candidate. It is not known whether natural exposure to Plasmodium falciparum stimulates HGXPRT T cell reactivity in humans.
Methods: PBMC and plasma collected from malaria-exposed Indonesians during infection and 7-28 days after anti-malarial therapy, were assessed for HGXPRT recognition using CFSE proliferation, IFNgamma ELISPOT assay and ELISA.
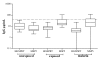
Results: HGXPRT-specific T cell proliferation was found in 44% of patients during acute infection; in 80% of responders both CD4+ and CD8+ T cell subsets proliferated. Antigen-specific T cell proliferation was largely lost within 28 days of parasite clearance. HGXPRT-specific IFN-gamma production was more frequent 28 days after treatment than during acute infection. HGXPRT-specific plasma IgG was undetectable even in individuals exposed to malaria for at least two years.
Conclusion: The prevalence of acute proliferative and convalescent IFNgamma responses to HGXPRT demonstrates cellular immunogenicity in humans. Further studies to determine minimal HGXPRT epitopes, the specificity of responses for Plasmodia and associations with protection are required. Frequent and robust T cell proliferation, high sequence conservation among Plasmodium species and absent IgG responses distinguish HGXPRT from other malaria antigens.
Figures




References
-
- Pombo DJ, Lawrence G, Hirunpetcharat C, Rzepczyk C, Bryden M, Cloonan N, Anderson K, Mahakunkijcharoen Y, Martin LB, Wilson D, et al. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet. 2002;360:610–617. doi: 10.1016/S0140-6736(02)09784-2. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

