A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses
- PMID: 19501001
- PMCID: PMC2764254
- DOI: 10.1016/j.immuni.2009.04.012
A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses
Abstract
Missense mutations of the gene encoding NLRP3 are associated with autoinflammatory disorders characterized with excessive production of interleukin-1beta (IL-1beta). Here we analyzed the immune responses of gene-targeted mice carrying a mutation in the Nlrp3 gene equivalent to the human mutation associated with Muckle-Wells Syndrome. We found that antigen-presenting cells (APCs) from such mice produced massive amounts of IL-1beta upon stimulation with microbial stimuli in the absence of ATP. This was likely due to a diminished inflammasome activation threshold that allowed a response to the small amount of agonist. Moreover, the Nlrp3 gene-targeted mice exhibited skin inflammation characterized by neutrophil infiltration and a Th17 cytokine-dominant response, which originated from hematopoietic cells. The inflammation of Nlrp3 gene-targeted mice resulted from excess IL-1beta production from APCs, which augmented Th17 cell differentiation. These results demonstrate that the NLRP3 mutation leads to inflammasome hyperactivation and consequently Th17 cell-dominant immunopathology in autoinflammation.
Conflict of interest statement
Figures
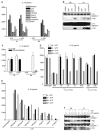

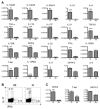

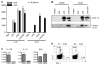

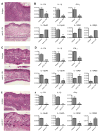
Comment in
-
Knocking in the NLRP3 inflammasome.Immunity. 2009 Jun 19;30(6):761-3. doi: 10.1016/j.immuni.2009.06.001. Immunity. 2009. PMID: 19538926
References
-
- Aksentijevich I, DP C, Remmers EF, Mueller JL, Le J, Kolodner RD, Moak Z, Chuang M, Austin F, Goldbach-Mansky R, et al. The clinical continuum of cryopyrinopathies: novel CIAS1 mutations in North American patients and a new cryopyrin model. Arthritis and rheumatism. 2007;56:1273–1285. - PMC - PubMed
-
- Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nature medicine. 2007;13:711–718. - PubMed
-
- Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. - PubMed
-
- Chabaud M, Fossiez F, Taupin JL, Miossec P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol. 1998;161:409–414. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

