THAP5 is a human cardiac-specific inhibitor of cell cycle that is cleaved by the proapoptotic Omi/HtrA2 protease during cell death
- PMID: 19502560
- PMCID: PMC2724199
- DOI: 10.1152/ajpheart.00234.2009
THAP5 is a human cardiac-specific inhibitor of cell cycle that is cleaved by the proapoptotic Omi/HtrA2 protease during cell death
Abstract
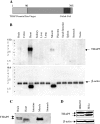
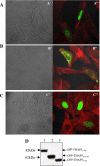
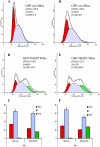
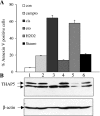
Omi/HtrA2 is a mitochondrial serine protease that has a dual function: while confined in the mitochondria, it promotes cell survival, but when released into the cytoplasm, it participates in caspase-dependent as well as caspase-independent cell death. To investigate the mechanism of Omi/HtrA2's function, we set out to isolate and characterize novel substrates for this protease. We have identified Thanatos-associated protein 5 (THAP5) as a specific interactor and substrate of Omi/HtrA2 in cells undergoing apoptosis. This protein is an uncharacterized member of the THAP family of proteins. THAP5 has a unique pattern of expression and is found predominantly in the human heart, although a very low expression is also seen in the human brain and muscle. THAP5 protein is localized in the nucleus and, when ectopically expressed, induces cell cycle arrest. During apoptosis, THAP5 protein is degraded, and this process can be blocked using a specific Omi/HtrA2 inhibitor, leading to reduced cell death. In patients with coronary artery disease, THAP5 protein levels substantially decrease in the myocardial infarction area, suggesting a potential role of this protein in human heart disease. This work identifies human THAP5 as a cardiac-specific nuclear protein that controls cell cycle progression. Furthermore, during apoptosis, THAP5 is cleaved and removed by the proapoptotic Omi/HtrA2 protease. Taken together, we provide evidence to support that THAP5 and its regulation by Omi/HtrA2 provide a new link between cell cycle control and apoptosis in cardiomyocytes.
Figures



References
-
- Adachi S, Ito H, Tamamori-Adachi M, Ono Y, Nozato T, Abe S, Ikeda M, Marumo F, Hiroe M. Cyclin A/cdk2 activation is involved in hypoxia-induced apoptosis in cardiomyocytes. Circ Res 88: 408–414, 2001. - PubMed
-
- Bhuiyan MS, Fukunaga K. Activation of HtrA2, a mitochondrial serine protease mediates apoptosis: current knowledge on HtrA2 mediated myocardial ischemia/reperfusion injury. Cardiovasc Ther 26: 224–232, 2008. - PubMed
-
- Bhuiyan MS, Fukunaga K. Inhibition of HtrA2/Omi ameliorates heart dysfunction following ischemia/reperfusion injury in rat heart in vivo. Eur J Pharmacol 557: 168–177, 2007. - PubMed
-
- Boxem M, van den Heuvel S. C. elegans class B synthetic multivulva genes act in G(1) regulation. Curr Biol 12: 906–911, 2002. - PubMed
-
- Boxem M, van den Heuvel S. lin-35 Rb and cki-1 Cip/Kip cooperate in developmental regulation of G1 progression in C. elegans. Development 128: 4349–4359, 2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

