Alternative splicing of Cav1.2 channel exons in smooth muscle cells of resistance-size arteries generates currents with unique electrophysiological properties
- PMID: 19502562
- PMCID: PMC2724194
- DOI: 10.1152/ajpheart.00109.2009
Alternative splicing of Cav1.2 channel exons in smooth muscle cells of resistance-size arteries generates currents with unique electrophysiological properties
Abstract
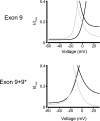
Voltage-dependent calcium (Ca(2+), Ca(V)1.2) channels are the primary Ca(2+) entry pathway in smooth muscle cells of resistance-size (myogenic) arteries, but their molecular identity remains unclear. Here we identified and quantified Ca(V)1.2 alpha(1)-subunit splice variation in myocytes of rat resistance-size (100-200 microm diameter) cerebral arteries. Full-length clones containing either exon 1b or the recently identified exon 1c exhibited additional primary splice variation at exons 9*, 21/22, 31/32, and +/- 33. Real-time PCR confirmed the findings from full-length clones and indicated that the major Ca(V)1.2 variant contained exons 1c, 8, 21, and 32+33, with approximately 57% containing 9*. Exon 9* was more prevalent in clones containing 1c (72%) than in those containing 1b (33%), suggesting exon-selective combinatorial splicing. To examine the functional significance of this splicing profile, membrane currents produced by each of the four exon 1b/c/ +/- 9* variants were characterized following transfection in HEK293 cells. Exon 1c and 9* caused similar hyperpolarizing shifts in both current-voltage relationships and voltage-dependent activation of currents. Furthermore, exon 9* induced a hyperpolarizing shift only in the voltage-dependent activation of channels containing exon 1b, but not in those containing exon 1c. In contrast, exon 1b, 1c, or +9* did not alter voltage-dependent inactivation. In summary, we have identified the Ca(V)1.2 alpha(1)-subunit splice variant population that is expressed in myocytes of resistance-size arteries and the unique electrophysiological properties of recombinant channels formed by exon 1 and 9* variation. The predominance of exon 1c and 9* in smooth muscle cell Ca(V)1.2 channels causes a hyperpolarizing shift in the voltage sensitivity of currents toward the physiological arterial voltage range.
Figures


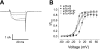
References
-
- Abernethy DR, Soldatov NM. Structure-functional diversity of human L-type Ca2+ channel: perspectives for new pharmacological targets. J Pharmacol Exp Ther 300: 724–728, 2002. - PubMed
-
- Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol 13: 298–307, 2003. - PubMed
-
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21, 2000. - PubMed
-
- Bogdanov Y, Brice NL, Canti C, Page KM, Li M, Volsen SG, Dolphin AC. Acidic motif responsible for plasma membrane association of the voltage-dependent calcium channel β1b subunit. Eur J Neurosci 12: 894–902, 2000. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

