Lower risk of resistance after short-course HAART compared with zidovudine/single-dose nevirapine used for prevention of HIV-1 mother-to-child transmission
- PMID: 19502990
- PMCID: PMC2765911
- DOI: 10.1097/QAI.0b013e3181aa8a22
Lower risk of resistance after short-course HAART compared with zidovudine/single-dose nevirapine used for prevention of HIV-1 mother-to-child transmission
Abstract
Background: Antiretroviral resistance after short-course regimens used to prevent mother-to-child transmission has consequences for later treatment. Directly comparing the prevalence of resistance after short-course regimens of highly active antiretroviral therapy (HAART) and zidovudine plus single-dose nevirapine (ZDV/sdNVP) will provide critical information when assessing the relative merits of these antiretroviral interventions.
Methods: In a clinical trial in Kenya, pregnant women were randomized to receive either ZDV/sdNVP or a short-course of HAART through 6 months of breastfeeding. Plasma samples were collected 3-12 months after treatment cessation, and resistance to reverse transcriptase inhibitors was assessed using both a sequencing assay and highly sensitive allele-specific polymerase chain reaction assays.
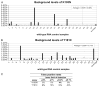
Results: No mutations associated with resistance were detectable by sequencing in either the ZDV/sdNVP or HAART arms at 3 months posttreatment, indicating that resistant viruses were not present in >20% of virus. Using allele-specific polymerase chain reaction assays for K103N and Y181C, we detected low levels of resistant virus in 75% of women treated with ZDV/sdNVP and only 18% of women treated with HAART (P = 0.007). Y181C was more prevalent than K103N at 3 months and showed little evidence of decay by 12 months.
Conclusions: Our finding provides evidence that compared with ZDV/sdNVP, HAART reduces but does not eliminate nevirapine resistance.
Figures
Comment in
-
Optimal versus suboptimal treatment for HIV-infected pregnant women and HIV-exposed infants in clinical research studies.J Acquir Immune Defic Syndr. 2009 Aug 15;51(5):509-12. doi: 10.1097/QAI.0b013e3181aa8a3d. J Acquir Immune Defic Syndr. 2009. PMID: 19521253 Review. No abstract available.
References
-
- World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infant: towards universal access. [Accessed July 17, 2006]. Available at: http://www.who.int/hiv/pub/guidelines/pmtctguidelines3.pdf. - PubMed
-
- Gray GE, Saloojee H. Breast-feeding, antiretroviral prophylaxis, and HIV. N Engl J Med. 2008;359:189–191. - PubMed
-
- Mofenson LM. Antiretroviral prophylaxis to reduce breast milk transmission of HIV type 1: new data but still questions. J Acquir Immune Defic Syndr. 2008;48:237–240. - PubMed
-
- Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med February 4. 2008;359:119–129. - PubMed
-
- Thomas T, Masaba R, Ndivo R, et al. Prevention of mother-to-child transmission of HIV-1 among breastfeeding mothers using HAART: the Kisumu Breastfeeding Study, Kisumu, Kenya, 2003–2007. Paper presented at: 15th Conference on Retroviruses and Opportunistic Infections; February 4, 2008; Boston, MA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

