Transmission and spreading of tauopathy in transgenic mouse brain
- PMID: 19503072
- PMCID: PMC2726961
- DOI: 10.1038/ncb1901
Transmission and spreading of tauopathy in transgenic mouse brain
Abstract
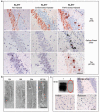
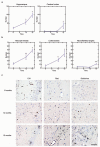
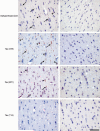
Hyperphosphorylated tau makes up the filamentous intracellular inclusions of several neurodegenerative diseases, including Alzheimer's disease. In the disease process, neuronal tau inclusions first appear in the transentorhinal cortex from where they seem to spread to the hippocampal formation and neocortex. Cognitive impairment becomes manifest when inclusions reach the hippocampus, with abundant neocortical tau inclusions and extracellular beta-amyloid deposits being the defining pathological hallmarks of Alzheimer's disease. An abundance of tau inclusions, in the absence of beta-amyloid deposits, defines Pick's disease, progressive supranuclear palsy, corticobasal degeneration and other diseases. Tau mutations cause familial forms of frontotemporal dementia, establishing that tau protein dysfunction is sufficient to cause neurodegeneration and dementia. Thus, transgenic mice expressing mutant (for example, P301S) human tau in nerve cells show the essential features of tauopathies, including neurodegeneration and abundant filaments made of hyperphosphorylated tau protein. By contrast, mouse lines expressing single isoforms of wild-type human tau do not produce tau filaments or show neurodegeneration. Here we have used tau-expressing lines to investigate whether experimental tauopathy can be transmitted. We show that injection of brain extract from mutant P301S tau-expressing mice into the brain of transgenic wild-type tau-expressing animals induces assembly of wild-type human tau into filaments and spreading of pathology from the site of injection to neighbouring brain regions.
Figures

References
-
- Goedert M, Spillantini MG. A century of Alzheimer's disease. Science. 2006;314:777–781. - PubMed
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
-
- Poorkaj P, et al. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann. Neurol. 1998;43:815–825. - PubMed
-
- Hutton M, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

