NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation
- PMID: 19503084
- PMCID: PMC2746760
- DOI: 10.1038/nn.2334
NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation
Abstract
Neuronal NMDA receptor (NMDAR) activation leads to the formation of superoxide, which normally acts in cell signaling. With extensive NMDAR activation, the resulting superoxide production leads to neuronal death. It is widely held that NMDA-induced superoxide production originates from the mitochondria, but definitive evidence for this is lacking. We evaluated the role of the cytoplasmic enzyme NADPH oxidase in NMDA-induced superoxide production. Neurons in culture and in mouse hippocampus responded to NMDA with a rapid increase in superoxide production, followed by neuronal death. These events were blocked by the NADPH oxidase inhibitor apocynin and in neurons lacking the p47(phox) subunit, which is required for NADPH oxidase assembly. Superoxide production was also blocked by inhibiting the hexose monophosphate shunt, which regenerates the NADPH substrate, and by inhibiting protein kinase C zeta, which activates the NADPH oxidase complex. These findings identify NADPH oxidase as the primary source of NMDA-induced superoxide production.
Figures
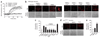
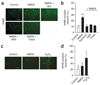

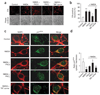

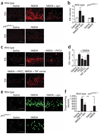
Comment in
-
Reactive oxygen species are NOXious for neurons.Nat Neurosci. 2009 Jul;12(7):819-20. doi: 10.1038/nn0709-819. Nat Neurosci. 2009. PMID: 19554046 No abstract available.
References
-
- Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide roduction and neurotoxicity. Nature. 1993;364:535–537. - PubMed
-
- MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL. NMDA receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature. 1986;321:519–522. - PubMed
-
- Klann E. Cell-permeable scavengers of superoxide prevent long-term potentiation in hippocampal area CA1. J. Neurophysiol. 1998;80:452–457. - PubMed
-
- MacDonald JF, Jackson MF, Beazely MA. Hippocampal long-term synaptic lasticity and signal amplification of NMDA receptors. Crit. Rev. Neurobiol. 2006;18:71–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

