HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction
- PMID: 19503085
- PMCID: PMC2701973
- DOI: 10.1038/nn.2333
HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction
Erratum in
-
Author Correction: HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the β-catenin-TCF interaction.Nat Neurosci. 2025 Aug;28(8):1810-1811. doi: 10.1038/s41593-025-02034-w. Nat Neurosci. 2025. PMID: 40670687 No abstract available.
Abstract
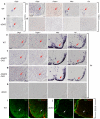
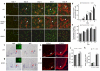
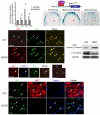
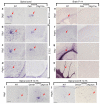
Oligodendrocyte development is regulated by the interaction of repressors and activators in a complex transcriptional network. We found that two histone-modifying enzymes, HDAC1 and HDAC2, were required for oligodendrocyte formation. Genetic deletion of both Hdac1 and Hdac2 in oligodendrocyte lineage cells resulted in stabilization and nuclear translocation of beta-catenin, which negatively regulates oligodendrocyte development by repressing Olig2 expression. We further identified the oligodendrocyte-restricted transcription factor TCF7L2/TCF4 as a bipartite co-effector of beta-catenin for regulating oligodendrocyte differentiation. Targeted disruption of Tcf7l2 in mice led to severe defects in oligodendrocyte maturation, whereas expression of its dominant-repressive form promoted precocious oligodendrocyte specification in developing chick neural tube. Transcriptional co-repressors HDAC1 and HDAC2 compete with beta-catenin for TCF7L2 interaction to regulate downstream genes involved in oligodendrocyte differentiation. Thus, crosstalk between HDAC1/2 and the canonical Wnt signaling pathway mediated by TCF7L2 serves as a regulatory mechanism for oligodendrocyte differentiation.
Figures


Comment in
-
Genetics meets epigenetics: HDACs and Wnt signaling in myelin development and regeneration.Nat Neurosci. 2009 Jul;12(7):815-7. doi: 10.1038/nn0709-815. Nat Neurosci. 2009. PMID: 19554044 No abstract available.
References
-
- Hsieh J, Gage FH. Chromatin remodeling in neural development and plasticity. Curr Opin Cell Biol. 2005;17:664–671. - PubMed
-
- Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Bio. 2004;338:17–31. - PubMed
-
- Cunliffe VT, Casaccia-Bonnefil P. Histone deacetylase 1 is essential for oligodendrocyte specification in the zebrafish CNS. Mech Dev. 2006;123:24–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

