Orally available Mn porphyrins with superoxide dismutase and catalase activities
- PMID: 19504132
- PMCID: PMC2716445
- DOI: 10.1007/s00775-009-0550-4
Orally available Mn porphyrins with superoxide dismutase and catalase activities
Abstract
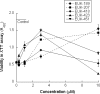
Superoxide dismutase/catalase mimetics, such as salen Mn complexes and certain metalloporphyrins, catalytically neutralize reactive oxygen and nitrogen species, which have been implicated in the pathogenesis of many serious diseases. Both classes of mimetic are protective in animal models of oxidative stress. However, only AEOL11207 and EUK-418, two uncharged Mn porphyrins, have been shown to be orally bioavailable. In this study, EUK-418 and several new analogs (the EUK-400 series) were synthesized and shown to exhibit superoxide dismutase, catalase, and peroxidase activities in vitro. Some also protected PC12 cells against staurosporine-induced cell death. All EUK-400 compounds were stable in simulated gastric fluid, and most were substantially more lipophilic than the salen Mn complexes EUK-189 and EUK-207, which lack oral activity. Pharmacokinetics studies demonstrate the presence of all EUK-400 series compounds in the plasma of rats after oral administration. These EUK-400 series compounds are potential oral therapeutic agents for cellular damage caused by oxidative stress.
Figures




Similar articles
-
Oxidation of nitric oxide by oxomanganese-salen complexes: a new mechanism for cellular protection by superoxide dismutase/catalase mimetics.Biochem J. 2002 Aug 15;366(Pt 1):97-107. doi: 10.1042/BJ20020154. Biochem J. 2002. PMID: 11994046 Free PMC article.
-
Novel synthetic SOD/catalase mimetics can mitigate capillary endothelial cell apoptosis caused by ionizing radiation.Radiat Res. 2010 Jun;173(6):748-59. doi: 10.1667/RR1948.1. Radiat Res. 2010. PMID: 20518654 Free PMC article.
-
Evaluation of the compounds commonly known as superoxide dismutase and catalase mimics in cellular models.J Inorg Biochem. 2021 Jun;219:111431. doi: 10.1016/j.jinorgbio.2021.111431. Epub 2021 Mar 19. J Inorg Biochem. 2021. PMID: 33798828
-
Salen Mn complexes mitigate radiation injury in normal tissues.Anticancer Agents Med Chem. 2011 May 1;11(4):359-72. doi: 10.2174/187152011795677490. Anticancer Agents Med Chem. 2011. PMID: 21453241 Free PMC article. Review.
-
Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential.Antioxid Redox Signal. 2010 Sep 15;13(6):877-918. doi: 10.1089/ars.2009.2876. Antioxid Redox Signal. 2010. PMID: 20095865 Free PMC article. Review.
Cited by
-
Design, mechanism of action, bioavailability and therapeutic effects of mn porphyrin-based redox modulators.Med Princ Pract. 2013;22(2):103-30. doi: 10.1159/000341715. Epub 2012 Oct 16. Med Princ Pract. 2013. PMID: 23075911 Free PMC article. Review.
-
A comprehensive evaluation of catalase-like activity of different classes of redox-active therapeutics.Free Radic Biol Med. 2015 Sep;86:308-21. doi: 10.1016/j.freeradbiomed.2015.05.018. Epub 2015 May 28. Free Radic Biol Med. 2015. PMID: 26026699 Free PMC article.
-
Glycoengineering artificial receptors for microglia to phagocytose Aβ aggregates.Chem Sci. 2021 Feb 23;12(13):4963-4969. doi: 10.1039/d0sc07067j. Chem Sci. 2021. PMID: 34163743 Free PMC article.
-
Striatal neuroprotection from neonatal hypoxia-ischemia in piglets by antioxidant treatment with EUK-134 or edaravone.Dev Neurosci. 2011;33(3-4):299-311. doi: 10.1159/000327243. Epub 2011 Jun 24. Dev Neurosci. 2011. PMID: 21701140 Free PMC article.
-
Pursuing the Elixir of Life: In Vivo Antioxidative Effects of Manganosalen Complexes.Antioxidants (Basel). 2020 Aug 10;9(8):727. doi: 10.3390/antiox9080727. Antioxidants (Basel). 2020. PMID: 32785017 Free PMC article. Review.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1080/09553000410001692726', 'is_inner': False, 'url': 'https://doi.org/10.1080/09553000410001692726'}, {'type': 'PubMed', 'value': '15204702', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15204702/'}]}
- Robbins MEC, Zhao W (2004) In J Radiat Biol 80:251–259 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0074-7742(07)82016-2', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0074-7742(07)82016-2'}, {'type': 'PubMed', 'value': '17678968', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17678968/'}]}
- Reynolds A, Laurie C, Lee Mosley R, Gendelman HE (2007) Int Rev Neurobiol 82:297–325 - PubMed
-
- None
- Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine. Oxford University Press, Oxford
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1021/jm020207y', 'is_inner': False, 'url': 'https://doi.org/10.1021/jm020207y'}, {'type': 'PubMed', 'value': '12238934', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12238934/'}]}
- Doctrow SR, Huffman K, Marcus CB, Tocco G, Malfroy E, Adinolfi CA, Kruk H, Baker K, Lazarowych N, Mascarenhas J, Malfroy B (2002) J Med Chem 45:4549–4558 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '8089112', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/8089112/'}]}
- Faulkner KM, Liochev SI, Fridovich I (1994) J Biol Chem 269:23471–23476 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

