Ca(2+) regulation of endocochlear potential in marginal cells
- PMID: 19504169
- PMCID: PMC10717738
- DOI: 10.1007/s12576-009-0043-9
Ca(2+) regulation of endocochlear potential in marginal cells
Abstract
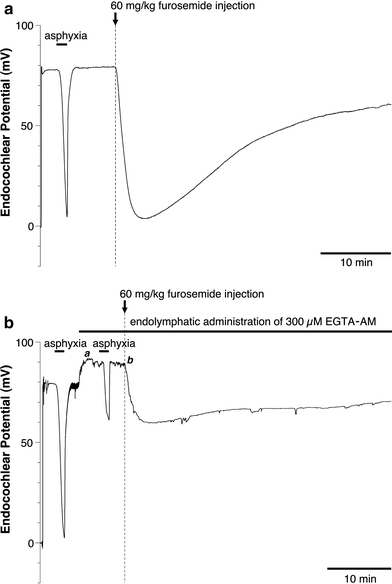
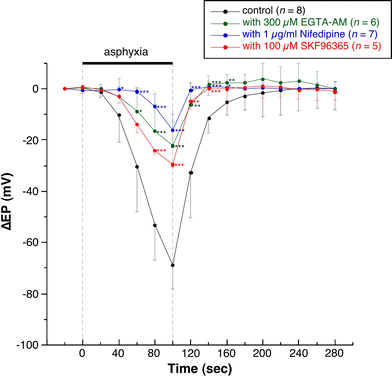
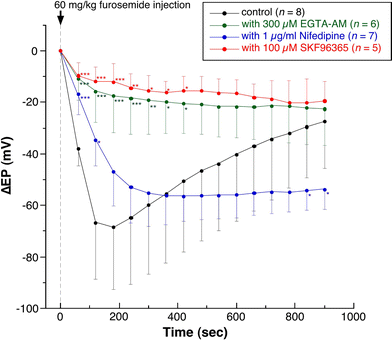
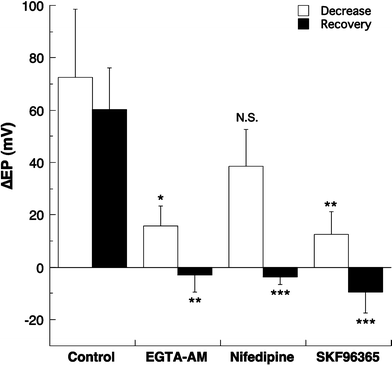
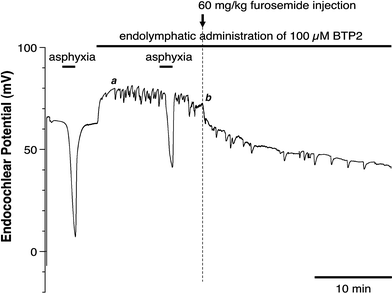
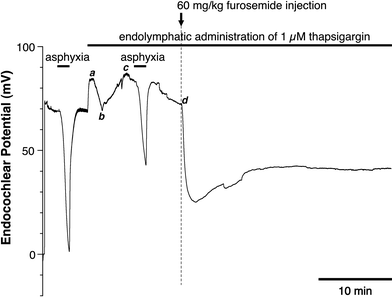
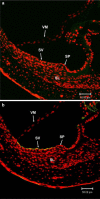
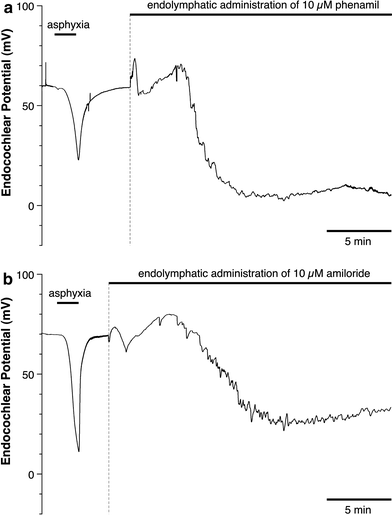
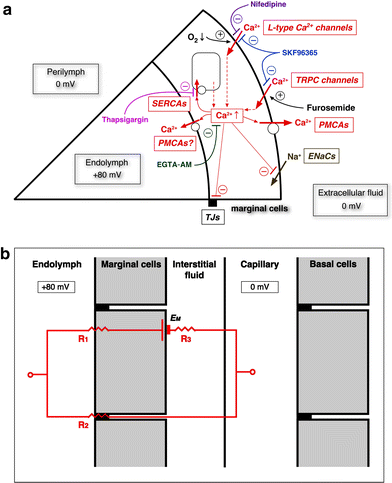
We examined the effect of the cytosolic Ca(2+) concentration ([Ca(2+)](c)) in marginal cells on the asphyxia- or furosemide-induced decrease in the endocochlear potential (EP) by perfusing the endolymph with or without a Ca(2+) chelator or inhibitors of Ca(2+)-permeable channels or Ca(2+)-pump during transient asphyxia or intravenous administration of furosemide. We obtained the following results. (1) Endolymphatic administration of SKF96365 (an inhibitor of TRPC and L-type Ca(2+) channels) or EGTA-acetoxymethyl ester (EGTA-AM) significantly inhibited both the transient asphyxia-induced decrease in EP (TAID) and the furosemide-induced decrease in EP (FUID). (2) Endolymphatic perfusion with nifedipine significantly inhibited the TAID but not the FUID. (3) The recovery from the FUID was significantly suppressed by perfusing the endolymph with EGTA-AM, nifedipine, or SKF96365. (4) Endolymphatic administration of thapsigargin inhibited both the FUID and TAID. (5) The recovery rate from the FUID was much slower than that from the TAID, indicating that furosemide may inhibit the Ca(2+)-pump. (6) A strong reaction in immunohistochemical staining for TRPC channels was observed in the luminal and basolateral membranes of marginal cells. (7) A positive staining reaction for the gamma subunit of epithelial Na(+) channels was observed in the luminal and basolateral membranes of marginal cells. (8) Positive EP was diminished toward 0 mV by the endolymphatic perfusion with 10 muM amiloride or 10 muM phenamil. Taken together, these findings suggest that [Ca(2+)](c) regulated by endoplasmic Ca(2+)-pump and Ca(2+)-permeable channels in marginal cells may regulate the positive EP, which is partly produced by the diffusion potential of Na(+) across the basolateral membrane in marginal cells.
Figures



Similar articles
-
How is the highly positive endocochlear potential formed? The specific architecture of the stria vascularis and the roles of the ion-transport apparatus.Pflugers Arch. 2010 Mar;459(4):521-33. doi: 10.1007/s00424-009-0754-z. Epub 2009 Dec 11. Pflugers Arch. 2010. PMID: 20012478 Review.
-
Endolymphatic perfusion with EGTA-acetoxymethyl ester inhibits asphyxia- and furosemide-induced decrease in endocochlear potential in guinea pigs.Jpn J Physiol. 2005 Feb;55(1):53-60. doi: 10.2170/jjphysiol.R2086. Jpn J Physiol. 2005. PMID: 15796789
-
Physiological role of L-type Ca2+ channels in marginal cells in the stria vascularis of guinea pigs.J Physiol Sci. 2007 Oct;57(5):287-98. doi: 10.2170/physiolsci.RP006807. Epub 2007 Oct 29. J Physiol Sci. 2007. PMID: 17963592
-
Effects of CO2/HCO3- in perilymph on the endocochlear potential in guinea pigs.J Physiol Sci. 2007 Feb;57(1):15-22. doi: 10.2170/physiolsci.RP012006. Epub 2006 Dec 15. J Physiol Sci. 2007. PMID: 17169167
-
Effects of a calcium channel blocker and calcium chelating agents on cochlear electrical activity in the guinea pig.Acta Otolaryngol. 1989 Jul-Aug;108(1-2):76-82. doi: 10.3109/00016488909107395. Acta Otolaryngol. 1989. PMID: 2504019
Cited by
-
CACHD1-deficient mice exhibit hearing and balance deficits associated with a disruption of calcium homeostasis in the inner ear.Hear Res. 2021 Sep 15;409:108327. doi: 10.1016/j.heares.2021.108327. Epub 2021 Aug 2. Hear Res. 2021. PMID: 34388681 Free PMC article.
-
Cellular mechanisms of noise-induced hearing loss.Hear Res. 2017 Jun;349:129-137. doi: 10.1016/j.heares.2016.11.013. Epub 2016 Dec 2. Hear Res. 2017. PMID: 27916698 Free PMC article.
-
How is the highly positive endocochlear potential formed? The specific architecture of the stria vascularis and the roles of the ion-transport apparatus.Pflugers Arch. 2010 Mar;459(4):521-33. doi: 10.1007/s00424-009-0754-z. Epub 2009 Dec 11. Pflugers Arch. 2010. PMID: 20012478 Review.
-
Single Cell and Single Nucleus RNA-Seq Reveal Cellular Heterogeneity and Homeostatic Regulatory Networks in Adult Mouse Stria Vascularis.Front Mol Neurosci. 2019 Dec 20;12:316. doi: 10.3389/fnmol.2019.00316. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31920542 Free PMC article.
-
Expression Patterns of Cav1.3 in the Developing Stria Vascularis of Sprague-Dawley Rats.J Int Adv Otol. 2025 Mar 25;21(2):1-6. doi: 10.5152/iao.2025.241581. J Int Adv Otol. 2025. PMID: 40206018 Free PMC article.
References
-
- Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. - PubMed
-
- Wangemann P, Nakaya K, Wu T, Maganti R, Erin MI, Sanneman J, Harbidge D, Billings S, Marcus DC. Loss of cochlear HCO3 − secretion causes deafness via endolymphatic acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model. Am J Physiol Renal Physiol. 2007;292:F1345–F1353. doi: 10.1152/ajprenal.00487.2006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

