Redox control of the cell cycle in health and disease
- PMID: 19505186
- PMCID: PMC2783918
- DOI: 10.1089/ars.2009.2513
Redox control of the cell cycle in health and disease
Abstract
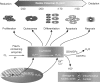
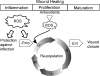
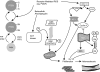
The cellular oxidation and reduction (redox) environment is influenced by the production and removal of reactive oxygen species (ROS). In recent years, several reports support the hypothesis that cellular ROS levels could function as ''second messengers'' regulating numerous cellular processes, including proliferation. Periodic oscillations in the cellular redox environment, a redox cycle, regulate cell-cycle progression from quiescence (G(0)) to proliferation (G(1), S, G(2), and M) and back to quiescence. A loss in the redox control of the cell cycle could lead to aberrant proliferation, a hallmark of various human pathologies. This review discusses the literature that supports the concept of a redox cycle controlling the mammalian cell cycle, with an emphasis on how this control relates to proliferative disorders including cancer, wound healing, fibrosis, cardiovascular diseases, diabetes, and neurodegenerative diseases. We hypothesize that reestablishing the redox control of the cell cycle by manipulating the cellular redox environment could improve many aspects of the proliferative disorders.
Figures







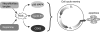

References
-
- Abergel RP. Pizzurro D. Meeker CA. Lask G. Matsuoka LY. Minor RR. Chu ML. Uitto J. Biochemical composition of the connective tissue in keloids and analysis of collagen metabolism in keloid fibroblast cultures. J Invest Dermatol. 1985;84:384–390. - PubMed
-
- AHA. American Heart Association. http://www.americanheart.org/presenter.jhtml?identifier=4478. [2009]. http://www.americanheart.org/presenter.jhtml?identifier=4478
-
- Albanese C. Johnson J. Watanabe G. Eklund N. Vu D. Arnold A. Pestell RG. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. - PubMed
-
- Aleem E. Kaldis P. Mouse models of cell cycle regulators: new paradigms. Results Probl Cell Differ. 2006;42:271–328. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

