Wnt5 is required for notochord cell intercalation in the ascidian Halocynthia roretzi
- PMID: 19505288
- PMCID: PMC2782306
- DOI: 10.1042/BC20090042
Wnt5 is required for notochord cell intercalation in the ascidian Halocynthia roretzi
Abstract
Background information: In the embryos of various animals, the body elongates after gastrulation by morphogenetic movements involving convergent extension. The Wnt/PCP (planar cell polarity) pathway plays roles in this process, particularly mediolateral polarization and intercalation of the embryonic cells. In ascidians, several factors in this pathway, including Wnt5, have been identified and found to be involved in the intercalation process of notochord cells.
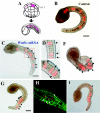
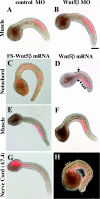
Results: In the present study, the role of the Wnt5 genes, Hr-Wnt5alpha (Halocynthia roretzi Wnt5alpha) and Hr-Wnt5beta, in convergent extension was investigated in the ascidian H. roretzi by injecting antisense oligonucleotides and mRNAs into single precursor blastomeres of various tissues, including notochord, at the 64-cell stage. Hr-Wnt5alpha is expressed in developing notochord and was essential for notochord morphogenesis. Precise quantitative control of its expression level was crucial for proper cell intercalation. Overexpression of Wnt5 proteins in notochord and other tissues that surround the notochord indicated that Wnt5alpha plays a role within the notochord, and is unlikely to be the source of polarizing cues arising outside the notochord. Detailed mosaic analysis of the behaviour of individual notochord cells overexpressing Wnt5alpha indicated that a Wnt5alpha-manipulated cell does not affect the behaviour of neighbouring notochord cells, suggesting that Wnt5alpha works in a cell-autonomous manner. This is further supported by comparison of the results of Wnt5alpha and Dsh (Dishevelled) knockdown experiments. In addition, our results suggest that the Wnt/PCP pathway is also involved in mediolateral intercalation of cells of the ventral row of the nerve cord (floor plate) and the endodermal strand.
Conclusion: The present study highlights the role of the Wnt5alpha signal in notochord convergent extension movements in ascidian embryos. Our results raise the novel possibility that Wnt5alpha functions in a cell-autonomous manner in activation of the Wnt/PCP pathway to polarize the protrusive activity that drives convergent extension.
Figures






References
-
- Akanuma T., Hori S., Darras S., Nishida H. Notch signaling is involved in neurogenesis in the ascidian embryos. Dev. Genes Evol. 2002;212:459–472. - PubMed
-
- Barrow J.R. Wnt/PCP signaling: a veritable polar star in establishing patterns of polarity in embryonic tissues. Semin. Cell Dev. Biol. 2006;17:185–193. - PubMed
-
- Christian J.L. BMP, Wnt and Hedgehog signals: how far can they go? Curr. Opin. Cell Biol. 2000;2:244–249. - PubMed
-
- Cloney R.A. Development of the ascidian notochord. Acta Embryol. Morphol. Exp. 1964;7:111–130.
-
- Fanto M., McNeill H. Planar polarity from flies to vertebrates. J. Cell Sci. 2004;117:527–533. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

