Simulation of the cost-effectiveness of malaria vaccines
- PMID: 19505328
- PMCID: PMC2701956
- DOI: 10.1186/1475-2875-8-127
Simulation of the cost-effectiveness of malaria vaccines
Abstract
Background: A wide range of possible malaria vaccines is being considered and there is a need to identify which vaccines should be prioritized for clinical development. An important element of the information needed for this prioritization is a prediction of the cost-effectiveness of potential vaccines in the transmission settings in which they are likely to be deployed. This analysis needs to consider a range of delivery modalities to ensure that clinical development plans can be aligned with the most appropriate deployment strategies.
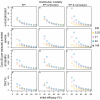
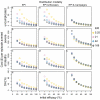
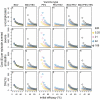
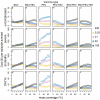
Methods: The simulations are based on a previously published individual-based stochastic model for the natural history and epidemiology of Plasmodium falciparum malaria. Three different vaccine types: pre-erythrocytic vaccines (PEV), blood stage vaccines (BSV), mosquito-stage transmission-blocking vaccines (MSTBV), and combinations of these, are considered each delivered via a range of delivery modalities (Expanded Programme of Immunization - EPI-, EPI with booster, and mass vaccination combined with EPI). The cost-effectiveness ratios presented are calculated for four health outcomes, for assumed vaccine prices of US$ 2 or US$ 10 per dose, projected over a 10-year period.
Results: The simulations suggest that PEV will be more cost-effective in low transmission settings, while BSV at higher transmission settings. Combinations of BSV and PEV are more efficient than PEV, especially in moderate to high transmission settings, while compared to BSV they are more cost-effective in moderate to low transmission settings. Combinations of MSTBV and PEV or PEV and BSV improve the effectiveness and the cost-effectiveness compared to PEV and BSV alone only when applied with EPI and mass vaccinations. Adding booster doses to the EPI is unlikely to be a cost-effective alternative to delivering vaccines via the EPI for any vaccine, while mass vaccination improves effectiveness, especially in low transmission settings, and is often a more efficient alternative to the EPI. However, the costs of increasing the coverage of mass vaccination over 50% often exceed the benefits.
Conclusion: The simulations indicate malaria vaccines might be efficient malaria control interventions, and that both transmission setting and vaccine delivery modality are important to their cost-effectiveness. Alternative vaccine delivery modalities to the EPI may be more efficient than the EPI. Mass vaccination is predicted to provide substantial health benefits at low additional costs, although achieving high coverage rates can lead to substantial incremental costs.
Figures

References
-
- Breman JG, Alilio M, Mills A. Conquering the intolerable burden of malaria: what's new, what's needed: a summary. Am J Trop Med Hyg. 2004;71:1–15. - PubMed
-
- Bojang KMP, Pinder M, Vigneron L, Alloueche A, Kester KE, Ballou WR, Conway D, Reece WHH, Gothard P, Yamuah L, Delchambre M, Voss G, Greenwood BM, Hill A, McAdam KP, Tornieporth N, Cohen JD, Doherty T. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet. 2001;358:1927–1934. doi: 10.1016/S0140-6736(01)06957-4. - DOI - PubMed
-
- Alonso P, Sacarlal J, Aponte J, Leach A, Macete E, Milman J, Mandomando I, Spiessens B, Guinovart C, Espasa M, et al. Efficacy of the RTS, S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–1420. doi: 10.1016/S0140-6736(04)17223-1. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

