Agricultural pesticide exposure and the molecular connection to lymphomagenesis
- PMID: 19506050
- PMCID: PMC2715093
- DOI: 10.1084/jem.20082842
Agricultural pesticide exposure and the molecular connection to lymphomagenesis
Abstract
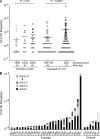
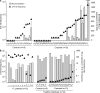
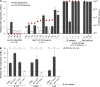
The t(14;18) translocation constitutes the initiating event of a causative cascade leading to follicular lymphoma (FL). t(14;18) translocations are present in blood from healthy individuals, but there is a trend of increased prevalence in farmers exposed to pesticides, a group recently associated with higher risk of t(14;18)(+) non-Hodgkin's lymphoma development. A direct connection between agricultural pesticide use, t(14;18) in blood, and malignant progression, however, has not yet been demonstrated. We followed t(14;18) clonal evolution over 9 yr in a cohort of farmers exposed to pesticides. We show that exposed individuals bear particularly high t(14;18) frequencies in blood because of a dramatic clonal expansion of activated t(14;18)(+) B cells. We further demonstrate that such t(14;18)(+) clones recapitulate the hallmark features of developmentally blocked FL cells, with some displaying aberrant activation-induced cytidine deaminase activity linked to malignant progression. Collectively, our data establish that expanded t(14;18)(+) clones constitute bona fide precursors at various stages of FL development, and provide a molecular connection between agricultural pesticide exposure, t(14;18) frequency in blood, and clonal progression.
Figures

References
-
- Aarts W.M., Bende R.J., Steenbergen E.J., Kluin P.M., Ooms E.C., Pals S.T., van Noesel C.J. 2000. Variable heavy chain gene analysis of follicular lymphomas: correlation between heavy chain isotype expression and somatic mutation load.Blood. 95:2922–2929 - PubMed
-
- Bende R.J., Smit L.A., van Noesel C.J. 2007. Molecular pathways in follicular lymphoma.Leukemia. 21:18–29 - PubMed
-
- Blair A., Zahm S.H., Pearce N.E., Heineman E.F., Fraumeni J.F. 1992. Clues to cancer etiology from studies of farmers.Scand. J. Work Environ. Health. 18:209–215 - PubMed
-
- Carlotti E., Wrench D., Matthews J., Iqbal S., Davies A., Norton A., Hart J., Lai R., Montoto S., Gribben J.G., et al. 2009. Transformation of follicular lymphoma to diffuse large B-cell lymphoma may occur by divergent evolution from a common progenitor cell or by direct evolution from the follicular lymphoma clone.Blood. 113:3553–3557 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

