Nukacin ISK-1, a bacteriostatic lantibiotic
- PMID: 19506061
- PMCID: PMC2715603
- DOI: 10.1128/AAC.01623-08
Nukacin ISK-1, a bacteriostatic lantibiotic
Abstract
We determined the mode of action of nukacin ISK-1. It did not cause membrane potential dissipation or the efflux of ATP or K(+) ions from the cells of a sensitive bacterial strain; however, it blocked the membrane depolarization activity of nisin. Nukacin ISK-1-treated cells had single arrangements of cells without the formation of a complete septum. A remarkable reduction in cell wall width was also observed, but cytoplasmic content was unaffected. We concluded that nukacin ISK-1 is bacteriostatic.
Figures
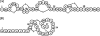



References
-
- Aso, Y., K. Okuda, J. Nagao, Y. Kamenasa, N. T. B. Phuong, H. Koga, K. Shioya, T. Sashihara, J. Nakayama, and K. Sonomoto. 2005. A novel type of immunity protein, NukH, for the lantibiotic nukacin ISK-1 produced by Staphylococcus warneri ISK-1. Biosci. Biotechnol. Biochem. 69:1403-1410. - PubMed
-
- Breukink, E., H. E. van Heusden, P. J. Vollmerhaus, E. Swiezewska, L. Brunner, S. Walker, A. J. Heck, and B. de Kruijff. 2003. Lipid II is an intrinsic component of the pore induced by nisin in bacterial membranes. J. Biol. Chem. 278:19898-19903. - PubMed
-
- Breukink, E., I. Wiedemann, C. van Kraaij, O. P. Kuipers, H.-G. Sahl, and B. de Kruijff. 1999. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361-2364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

