Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout
- PMID: 19506252
- PMCID: PMC2700910
- DOI: 10.1073/pnas.0901249106
Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout
Abstract
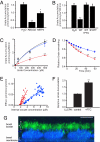
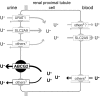
Genome-wide association studies (GWAS) have successfully identified common single nucleotide polymorphisms (SNPs) associated with a wide variety of complex diseases, but do not address gene function or establish causality of disease-associated SNPs. We recently used GWAS to identify SNPs in a genomic region on chromosome 4 that associate with serum urate levels and gout, a consequence of elevated urate levels. Here we show using functional assays that human ATP-binding cassette, subfamily G, 2 (ABCG2), encoded by the ABCG2 gene contained in this region, is a hitherto unknown urate efflux transporter. We further show that native ABCG2 is located in the brush border membrane of kidney proximal tubule cells, where it mediates renal urate secretion. Introduction of the mutation Q141K encoded by the common SNP rs2231142 by site-directed mutagenesis resulted in 53% reduced urate transport rates compared to wild-type ABCG2 (P < 0.001). Data from a population-based study of 14,783 individuals support rs2231142 as the causal variant in the region and show highly significant associations with urate levels [whites: P = 10(-30), minor allele frequency (MAF) 0.11; blacks P = 10(-4), MAF 0.03] and gout (adjusted odds ratio 1.68 per risk allele, both races). Our data indicate that at least 10% of all gout cases in whites are attributable to this causal variant. With approximately 3 million US individuals suffering from often insufficiently treated gout, ABCG2 represents an attractive drug target. Our study completes the chain of evidence from association to causation and supports the common disease-common variant hypothesis in the etiology of gout.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
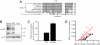

References
Publication types
MeSH terms
Substances
Grants and funding
- N01 HC055016/HL/NHLBI NIH HHS/United States
- N01 HC055019/HL/NHLBI NIH HHS/United States
- R01HL59367/HL/NHLBI NIH HHS/United States
- UL1 RR025005/RR/NCRR NIH HHS/United States
- R01 DK032753/DK/NIDDK NIH HHS/United States
- N01-HC-55016/HC/NHLBI NIH HHS/United States
- R01 HL086694/HL/NHLBI NIH HHS/United States
- N01 HC055015/HL/NHLBI NIH HHS/United States
- U01HG004402/HG/NHGRI NIH HHS/United States
- N01 HC055020/HL/NHLBI NIH HHS/United States
- N01-HC-55019/HC/NHLBI NIH HHS/United States
- R01HL087641/HL/NHLBI NIH HHS/United States
- N01-HC-55015/HC/NHLBI NIH HHS/United States
- N01-HC-55020/HC/NHLBI NIH HHS/United States
- N01 HC055018/HL/NHLBI NIH HHS/United States
- N01 HC055022/HL/NHLBI NIH HHS/United States
- R01HL086694/HL/NHLBI NIH HHS/United States
- UL1RR025005/RR/NCRR NIH HHS/United States
- R01DK32753/DK/NIDDK NIH HHS/United States
- N01-HC-55022/HC/NHLBI NIH HHS/United States
- R01 HL059367/HL/NHLBI NIH HHS/United States
- N01 HC055021/HL/NHLBI NIH HHS/United States
- N01-HC-55021/HC/NHLBI NIH HHS/United States
- HHSN268200625226C/PHS HHS/United States
- U01 HG004402/HG/NHGRI NIH HHS/United States
- N01-HC-55018/HC/NHLBI NIH HHS/United States
- R01 HL087641/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

