Tumor-associated macrophages infiltrate plasmacytomas and can serve as cell carriers for oncolytic measles virotherapy of disseminated myeloma
- PMID: 19507209
- PMCID: PMC2805200
- DOI: 10.1002/ajh.21444
Tumor-associated macrophages infiltrate plasmacytomas and can serve as cell carriers for oncolytic measles virotherapy of disseminated myeloma
Abstract
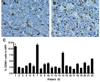
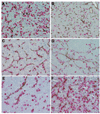
In multiple myeloma, some of the neoplastic plasma cells are diffusely dispersed among the normal bone marrow cells (bone marrow resident), whereas others are located in discrete, well-vascularized solid tumors (plasmacytomas) that may originate in bone or soft tissue. Interactions between bone marrow-resident myeloma cells and bone marrow stromal cells (BMSCs) are important determinants of myeloma pathogenesis. However, little is known of the factors sustaining myeloma growth and cell viability at the centers of expanding plasmacytomas, where there are no BMSCs. Histologic sections of 22 plasmacytomas from myeloma patients were examined after immunostaining. Abundant CD68+, CD163+, S100-negative macrophage infiltrates were identified in all tumors, accompanied by scattered collections of CD3+ T lymphocytes. The CD68+ tumor-associated macrophages (TAM) accounted for 2-12% of nucleated cells and were evenly distributed through the parenchyma. The TAM generally had dendritic morphology, and each dendrite was in close contact with multiple plasma cells. In some cases, the TAM were strikingly clustered around CD34+ blood vessels. To determine whether cells of the monocytic lineage might be exploitable as carriers for delivery of therapeutic agents to plasmacytomas, primary human CD14+ cells were infected with oncolytic measles virus and administered intravenously to mice bearing KAS6/1 human myeloma xenografts. The cell carriers localized to KAS6/1 tumors, where they transferred MV infection to myeloma cells and prolonged the survival of mice bearing disseminated human myeloma disease. Thus, TAM are a universal stromal component of the plasmacytomas of myeloma patients and may offer a promising new target for therapeutic exploitation. Am. J. Hematol. 2009. (c) 2009 Wiley-Liss, Inc.
Conflict of interest statement
Conflict of interest: Nothing to report.
Figures



Similar articles
-
Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies.Gene Ther. 2007 Feb;14(4):324-33. doi: 10.1038/sj.gt.3302880. Epub 2006 Oct 19. Gene Ther. 2007. PMID: 17051248
-
Remission of disseminated cancer after systemic oncolytic virotherapy.Mayo Clin Proc. 2014 Jul;89(7):926-33. doi: 10.1016/j.mayocp.2014.04.003. Epub 2014 May 14. Mayo Clin Proc. 2014. PMID: 24835528 Free PMC article.
-
Systemic therapy of disseminated myeloma in passively immunized mice using measles virus-infected cell carriers.Mol Ther. 2010 Jun;18(6):1155-64. doi: 10.1038/mt.2010.43. Epub 2010 Mar 16. Mol Ther. 2010. PMID: 20234340 Free PMC article.
-
Cell carriers to deliver oncolytic viruses to sites of myeloma tumor growth.Gene Ther. 2008 May;15(10):797-806. doi: 10.1038/gt.2008.45. Epub 2008 Mar 20. Gene Ther. 2008. PMID: 18356812 Review.
-
Oncolytic virotherapy for multiple myeloma.Expert Opin Biol Ther. 2008 Apr;8(4):463-73. doi: 10.1517/14712598.8.4.463. Expert Opin Biol Ther. 2008. PMID: 18352850 Review.
Cited by
-
Enhanced Delivery of Oncolytic Adenovirus by Neural Stem Cells for Treatment of Metastatic Ovarian Cancer.Mol Ther Oncolytics. 2018 Dec 13;12:79-92. doi: 10.1016/j.omto.2018.12.003. eCollection 2019 Mar 29. Mol Ther Oncolytics. 2018. PMID: 30719498 Free PMC article.
-
Quantitative Temporal in Vivo Proteomics Deciphers the Transition of Virus-Driven Myeloid Cells into M2 Macrophages.J Proteome Res. 2017 Sep 1;16(9):3391-3406. doi: 10.1021/acs.jproteome.7b00425. Epub 2017 Aug 23. J Proteome Res. 2017. PMID: 28768414 Free PMC article.
-
Potential and clinical translation of oncolytic measles viruses.Expert Opin Biol Ther. 2017 Mar;17(3):353-363. doi: 10.1080/14712598.2017.1288713. Expert Opin Biol Ther. 2017. PMID: 28129716 Free PMC article. Review.
-
Systemic Delivery Strategies for Oncolytic Viruses: Advancing Targeted and Efficient Tumor Therapy.Int J Mol Sci. 2025 Jul 18;26(14):6900. doi: 10.3390/ijms26146900. Int J Mol Sci. 2025. PMID: 40725148 Free PMC article. Review.
-
Cell carriers for oncolytic viruses: current challenges and future directions.Oncolytic Virother. 2013 Oct 9;2:47-56. doi: 10.2147/OV.S36623. eCollection 2013. Oncolytic Virother. 2013. PMID: 27512657 Free PMC article. Review.
References
-
- Kyle RA. Multiple myeloma: An odyssey of discovery. Br J Haematol. 2000;111:1035–1044. - PubMed
-
- Rosinol L, Blade J, Esteve J, et al. Smoldering multiple myeloma: Natural history and recognition of an evolving type. Br J Haematol. 2003;123:631–636. - PubMed
-
- Said JW, Rettig MR, Heppner K, et al. Localization of Kaposi’s sarcoma-associated herpesvirus in bone marrow biopsy samples from patients with multiple myeloma. Blood. 1997;90:4278–4282. - PubMed
-
- Hideshima T, Mitsiades C, Tonon G, et al. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
