Bacterial type IV secretion systems in human disease
- PMID: 19508287
- PMCID: PMC2784931
- DOI: 10.1111/j.1365-2958.2009.06751.x
Bacterial type IV secretion systems in human disease
Abstract
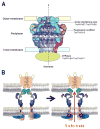
Type IV secretion (T4S) systems are versatile machines involved in many processes relevant to bacterial virulence, such as horizontal DNA transfer and effector translocation into human cells. A recent workshop organized by the International University of Andalousia in Baeza, Spain, covered most aspects of bacterial T4S relevant to human disease, ranging from the structural and mechanistic analysis of the T4S systems to the physiological roles of the translocated effector proteins in subverting cellular functions in infected humans. This review reports the highlights from this workshop, which include the first visualization of a T4S system core complex spanning both membranes of Gram-negative bacteria, the identification of the first host receptors for T4S systems, the identification and characterization of novel T4S effector proteins, the analysis of the molecular function of effector proteins in subverting human cellular functions and an analysis of the role of T4S systems in the evolution of pathogenic bacteria. Our increasing knowledge of the biology of T4S systems improves our ability to exploit them as biotechnological tools or to use them as novel targets for a new generation of antimicrobials.
Figures
References
-
- Babic A, Lindner AB, Vulic M, Stewart EJ, Radman M. Direct visualization of horizontal gene transfer. Science. 2008;319:1533–1536. - PubMed
-
- Backert S, Fronzes R, Waksman G. VirB2 and VirB5 proteins: specialized adhesins in bacterial type-IV secretion systems? Trends Microbiol. 2008;16:409–413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

