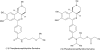Dual PDE3/4 inhibitors as therapeutic agents for chronic obstructive pulmonary disease
- PMID: 19508401
- PMCID: PMC2737648
- DOI: 10.1111/j.1476-5381.2009.00170.x
Dual PDE3/4 inhibitors as therapeutic agents for chronic obstructive pulmonary disease
Abstract
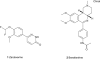
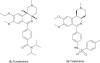
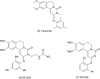
Phosphodiesterase (PDE)4, and to a lesser extent, PDE3/4 inhibitors have attracted considerable interest as potential therapeutic agents for diseases including chronic obstructive pulmonary disease. Indeed, ibudilast and theophylline are utilized clinically, and roflumilast is in late-stage clinical development. Unfortunately, however many PDE4 and dual PDE3/4 inhibitors have failed in early development due to low therapeutic ratios. The majority of these compounds are however orally administered and non-selective for either PDE3(A, B) or PDE4(A, B, C, D) subtypes. Developing an inhaled dual PDE3/4 inhibitor with subtype specificity may represent one strategy to improve the therapeutic index. Indeed combined inhibition of PDE3 and PDE4 inhibitor has additive and synergistic anti-inflammatory and bronchodilatory effects versus inhibition of either PDE3 or PDE4 alone. Given that synergy has been seen in terms of efficacy end points, an obvious concern is that synergy may also be observed in side effects. Interestingly, however, no synergy or additive effects with a combination of a PDE3 and PDE4 inhibitor in a cardiomyocyte assay were observed. This review will summarize the rationale for developing an inhaled dual PDE3/4 inhibitor, as a treatment for chronic obstructive pulmonary disease together with recent advances in trying to understand the pathogenesis of PDE inhibitor-induced mesenteric vasculitis (a key potential dose-limiting side effect of these agents), highlighting potential early and sensitive predictive biomarkers.
Figures
References
-
- Ariga M, Neitzert B, Nakae S, Mottin G, Bertrand C, Pruniaux MP, et al. Non-redundant function of phosphodiesterase 4D and 4B in neutrophil recruitment to the site of inflammation. J Immunol. 2004;173:7531–7538. - PubMed
-
- Barber R, Baillie GS, Bergmann R, Shepherd MC, Sepper R, Houslay MD, et al. Differential expression of PDE4 cAMP phosphodiesterase isoforms in inflammatory cells of smokers with COPD, smokers without COPD, and nonsmokers. Am J Physiol Lung Cell Mol Physiol. 2004;287:L332–L343. - PubMed
-
- Barnes PJ. New concepts in chronic obstructive pulmonary disease. Annu Rev Med. 2003;54:113–129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical