A 13-hour laboratory school study of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder
- PMID: 19508731
- PMCID: PMC2704174
- DOI: 10.1186/1753-2000-3-17
A 13-hour laboratory school study of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder
Abstract
Background: Lisdexamfetamine dimesylate (LDX) is indicated for the treatment of attention-deficit/hyperactivity disorder (ADHD) in children 6 to 12 years of age and in adults. In a previous laboratory school study, LDX demonstrated efficacy 2 hours postdose with duration of efficacy through 12 hours. The current study further characterizes the time course of effect of LDX.
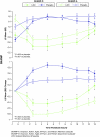
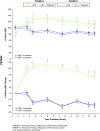
Methods: Children aged 6 to 12 years with ADHD were enrolled in a laboratory school study. The multicenter study consisted of open-label, dose-optimization of LDX (30, 50, 70 mg/d, 4 weeks) followed by a randomized, placebo-controlled, 2-way crossover phase (1 week each). Efficacy measures included the SKAMP (deportment [primary] and attention [secondary]) and PERMP (attempted/correct) scales (secondary) measured at predose and at 1.5, 2.5, 5, 7.5, 10, 12, and 13 hours postdose. Safety measures included treatment-emergent adverse events (AEs), physical examination, vital signs, and ECGs.
Results: A total of 117 subjects were randomized and 111 completed the study. Compared with placebo, LDX demonstrated significantly greater efficacy at each postdose time point (1.5 hours to 13.0 hours), as measured by SKAMP deportment and attention scales and PERMP (P < .005). The most common treatment-emergent AEs during dose optimization were decreased appetite (47%), insomnia (27%), headache (17%), irritability (16%), upper abdominal pain (16%), and affect lability (10%), which were less frequent in the crossover phase (6%, 4%, 5%, 1%, 2%, and 0% respectively).
Conclusion: In school-aged children (6 to 12 years) with ADHD, efficacy of LDX was maintained from the first time point (1.5 hours) up to the last time point assessed (13.0 hours). LDX was generally well tolerated, resulting in typical stimulant AEs.
Trial registration: Official Title: A Phase IIIb, Randomized, Double-Blind, Multi-Center, Placebo-Controlled, Dose-Optimization, Cross-Over, Analog Classroom Study to Assess the Time of Onset of Vyvanse (Lisdexamfetamine Dimesylate) in Pediatric Subjects Aged 6-12 With Attention-Deficit/Hyperactivity Disorder. ClinicalTrials.gov Identifier: NCT00500149 http://clinicaltrials.gov/ct2/show/NCT00500149.
Figures

References
-
- Pliszka SR, Crismon ML, Hughes CW, Conners CK, Emslie GJ, Jensen PS, McCracken JT, Swanson JM, Lopez M, the Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention-Deficit/Hyperactivity Disorder The Texas Children's Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45:642–657. doi: 10.1097/01.chi.0000215326.51175.eb. - DOI - PubMed
-
- Dulcan M, the Work Group on Quality Issues Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36:85S–121S. - PubMed
-
- Stein MA, Baren M. Welcome progress in the diagnosis and treatment of ADHD in adolescence. Contemp Pediatr. 2003;20:83–110.

