Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9
- PMID: 19509016
- PMCID: PMC2712800
- DOI: 10.2337/db09-0317
Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9
Abstract
Objective: Mesenchymal stem cells (MSCs) are known to be capable of suppressing immune responses, but the molecular mechanisms involved and the therapeutic potential of MSCs remain to be clarified.
Research design and methods: We investigated the molecular mechanisms underlying the immunosuppressive effects of MSCs in vitro and in vivo.
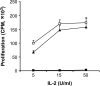
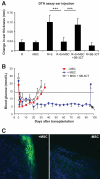
Results: Our results demonstrate that matrix metalloproteinases (MMPs) secreted by MSCs, in particular MMP-2 and MMP-9, play an important role in the suppressive activity of MSCs by reducing surface expression of CD25 on responding T-cells. Blocking the activity of MMP-2 and MMP-9 in vitro completely abolished the suppression of T-cell proliferation by MSCs and restored T-cell expression of CD25 as well as responsiveness to interleukin-2. In vivo, administration of MSCs significantly reduced delayed-type hypersensitivity responses to allogeneic antigen and profoundly prolonged the survival of fully allogeneic islet grafts in transplant recipients. Significantly, these MSC-mediated protective effects were completely reversed by in vivo inhibition of MMP-2 and MMP-9.
Conclusions: We demonstrate that MSCs can prevent islet allograft rejection leading to stable, long-term normoglycemia. In addition, we provide a novel insight into the mechanism underlying the suppressive effects of MSCs on T-cell responses to alloantigen.
Figures



Comment in
-
Mesenchymal stem cells: a potential border patrol for transplanted islets?Diabetes. 2009 Aug;58(8):1728-9. doi: 10.2337/db09-0749. Diabetes. 2009. PMID: 19638531 Free PMC article. No abstract available.
References
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR: Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143– 147 - PubMed
-
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB: Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res 2000; 61: 364– 370 - PubMed
-
- Zhang Y, Li C, Jiang X, Zhang S, Wu Y, Liu B, Tang P, Mao N: Human placenta-derived mesenchymal progenitor cells support culture expansion of long-term culture-initiating cells from cord blood CD34+ cells. Exp Hematol 2004; 32: 657– 664 - PubMed
-
- Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL: Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res 2000; 9: 841– 848 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

