SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states
- PMID: 19509029
- PMCID: PMC2741348
- DOI: 10.1093/cvr/cvp190
SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states
Abstract
Aims: Cardiac myocytes depend on a delicate balance of glucose and free fatty acids as energy sources, a balance that is disrupted in pathological states such as diabetic cardiomyopathy and myocardial ischaemia. There are two families of cellular glucose transporters: the facilitated-diffusion glucose transporters (GLUT); and the sodium-dependent glucose transporters (SGLT). It has long been thought that only the GLUT isoforms, GLUT1 and GLUT4, are responsible for cardiac myocyte glucose uptake. However, we discovered that one SGLT isoform, SGLT1, is also an important glucose transporter in heart. In this study, we aimed to determine the human and murine cardiac expression pattern of SGLT1 in health and disease and to determine its regulation.
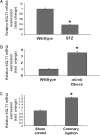
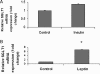
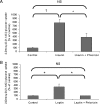
Methods and results: SGLT1 was largely localized to the cardiac myocyte sarcolemma. Changes in SGLT1 expression were observed in disease states in both humans and mouse models. SGLT1 expression was upregulated two- to three-fold in type 2 diabetes mellitus and myocardial ischaemia (P < 0.05). In humans with severe heart failure, functional improvement following implantation of left ventricular assist devices led to a two-fold increase in SGLT1 mRNA (P < 0.05). Acute administration of leptin to wildtype mice increased cardiac SGLT1 expression approximately seven-fold (P < 0.05). Insulin- and leptin-stimulated cardiac glucose uptake was significantly (P < 0.05) inhibited by phlorizin, a specific SGLT1 inhibitor.
Conclusion: Our data suggest that cardiac SGLT1 expression and/or function are regulated by insulin and leptin, and are perturbed in disease. This is the first study to examine the regulation of cardiac SGLT1 expression by insulin and leptin and to determine changes in SGLT1 expression in cardiac disease.
Figures

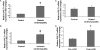
References
-
- Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. - PubMed
-
- Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovasc Res. 1997;33:243–257. - PubMed
-
- Schwenk RW, Luiken JJ, Bonen A, Glatz JF. Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc Res. 2008;79:249–258. - PubMed
-
- Wright EM, Hirsch JR, Loo DD, Zampighi GA. Regulation of Na+/glucose cotransporters. J Exp Biol. 1997;200:287–293. - PubMed
-
- Turk E, Zabel B, Mundlos S, Dyer J, Wright EM. Glucose/galactose malabsorption caused by a defect in the Na+/glucose cotransporter. Nature. 1991;350:354–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

