Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication
- PMID: 19509134
- PMCID: PMC4821072
- DOI: 10.1158/1078-0432.CCR-09-0334
Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication
Abstract
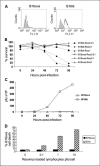
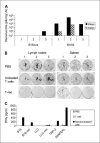
Purpose: Reovirus is a naturally occurring oncolytic virus in clinical trials. Although tumor infection by reovirus can generate adaptive antitumor immunity, its therapeutic importance versus direct viral oncolysis is undefined. This study addresses the requirement for viral oncolysis and replication, and the relative importance of antitumor immunity and direct oncolysis in therapy.
Experimental design: Nonantigen specific T cells loaded with reovirus were delivered i.v. to C57BL/6 and severe combined immunodeficient mice bearing lymph node and splenic metastases from the murine melanoma, B16ova, with assessment of viral replication, metastatic clearance by tumor colony outgrowth, and immune priming. Human cytotoxic lymphocyte priming assays were done with dendritic cells loaded with Mel888 cells before the addition of reovirus.
Results: B16ova was resistant to direct oncolysis in vitro, and failed to support reovirus replication in vitro or in vivo. Nevertheless, reovirus purged lymph node and splenic metastases in C57BL/6 mice and generated antitumor immunity. In contrast, reovirus failed to reduce tumor burden in severe combined immunodeficient mice bearing either B16ova or reovirus-sensitive B16tk metastases. In the human system, reovirus acted solely as an adjuvant when added to dendritic cells already loaded with Mel888, supporting priming of specific antitumor cytotoxic lymphocyte, in the absence of significant direct tumor oncolysis; UV-treated nonreplicating reovirus was similarly immunogenic.
Conclusion: The immune response is critical in mediating the efficacy of reovirus, and does not depend upon direct viral oncolysis or replication. The findings are of direct relevance to fulfilling the potential of this novel anticancer agent.
Figures



Similar articles
-
Tumor infection by oncolytic reovirus primes adaptive antitumor immunity.Clin Cancer Res. 2008 Nov 15;14(22):7358-66. doi: 10.1158/1078-0432.CCR-08-0831. Clin Cancer Res. 2008. PMID: 19010851 Free PMC article.
-
Dendritic cells and T cells deliver oncolytic reovirus for tumour killing despite pre-existing anti-viral immunity.Gene Ther. 2009 May;16(5):689-99. doi: 10.1038/gt.2009.29. Epub 2009 Mar 12. Gene Ther. 2009. PMID: 19282847 Free PMC article.
-
Reovirus virotherapy overrides tumor antigen presentation evasion and promotes protective antitumor immunity.Mol Cancer Ther. 2010 Nov;9(11):2924-33. doi: 10.1158/1535-7163.MCT-10-0590. Epub 2010 Oct 26. Mol Cancer Ther. 2010. PMID: 20978162
-
Reovirus and tumor oncolysis.J Microbiol. 2007 Jun;45(3):187-92. J Microbiol. 2007. PMID: 17618222 Review.
-
Role of Myeloid Cells in Oncolytic Reovirus-Based Cancer Therapy.Viruses. 2021 Apr 10;13(4):654. doi: 10.3390/v13040654. Viruses. 2021. PMID: 33920168 Free PMC article. Review.
Cited by
-
Multifaceted therapeutic targeting of ovarian peritoneal carcinomatosis through virus-induced immunomodulation.Mol Ther. 2013 Feb;21(2):338-47. doi: 10.1038/mt.2012.228. Epub 2012 Nov 13. Mol Ther. 2013. PMID: 23299799 Free PMC article.
-
Effectiveness and safety of pelareorep plus chemotherapy versus chemotherapy alone for advanced solid tumors: a meta-analysis.Front Pharmacol. 2023 Sep 26;14:1228225. doi: 10.3389/fphar.2023.1228225. eCollection 2023. Front Pharmacol. 2023. PMID: 37829303 Free PMC article.
-
Thunder and lightning: immunotherapy and oncolytic viruses collide.Mol Ther. 2011 Jun;19(6):1008-16. doi: 10.1038/mt.2011.65. Epub 2011 Apr 19. Mol Ther. 2011. PMID: 21505424 Free PMC article. Review.
-
A proapoptotic peptide derived from reovirus outer capsid protein {micro}1 has membrane-destabilizing activity.J Virol. 2011 Feb;85(4):1507-16. doi: 10.1128/JVI.01876-10. Epub 2010 Nov 24. J Virol. 2011. PMID: 21106751 Free PMC article.
-
Oncolytic virus-initiated protective immunity against prostate cancer.Mol Ther. 2011 Apr;19(4):797-804. doi: 10.1038/mt.2010.297. Epub 2011 Jan 18. Mol Ther. 2011. PMID: 21245852 Free PMC article.
References
-
- Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–76. - PubMed
-
- Aghi M, Martuza RL. Oncolytic viral therapies - the clinical experience. Oncogene. 2005;24:7802–16. - PubMed
-
- van Houdt WJ, Smakman N, van den Wollenberg DJ, et al. Transient infection of freshly isolated human colorectal tumor cells by reovirusT3D intermediate subviral particles. Cancer GeneTher. 2008;15:284–92. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

