Anti-EMMPRIN monoclonal antibody as a novel agent for therapy of head and neck cancer
- PMID: 19509148
- PMCID: PMC2796106
- DOI: 10.1158/1078-0432.CCR-09-0212
Anti-EMMPRIN monoclonal antibody as a novel agent for therapy of head and neck cancer
Abstract
Purpose: Extracellular matrix metalloprotease inducer (EMMPRIN) is a tumor surface protein that promotes growth and is overexpressed in head and neck cancer. These features make it a potential therapeutic target for monoclonal antibody (mAb)-based therapy. Because molecular therapy is considered more effective when delivered with conventional cytotoxic agents, anti-EMMPRIN therapy was assessed alone and in combination with external beam radiation.
Experimental design: Using a murine flank model, loss of EMMPRIN function was achieved by transfection with a small interfering RNA against EMMPRIN or treatment with a chimeric anti-EMMPRIN blocking mAb. Cytokine expression was assessed for xenografts, tumor cells, fibroblasts, and endothelial cells.
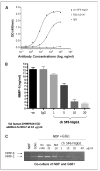
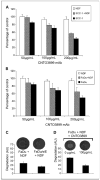
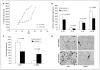
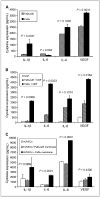
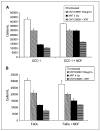
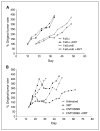
Results: Animals treated with anti-EMMPRIN mAb had delayed tumor growth compared with untreated controls, whereas treatment with combination radiation and anti-EMMPRIN mAb showed the greatest reduction in tumor growth (P = 0.001). Radiation-treated EMMPRIN knockdown xenografts showed a reduction in tumor growth compared with untreated knockdown controls (P = 0.01), whereas radiation-treated EMMPRIN-expressing xenografts did not show a delay in tumor growth. Immunohistochemical evaluation for Ki67 and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotin nick end labeling (TUNEL) resulted in a reduction in proliferation (P = 0.007) and increased apoptosis in anti-EMMPRIN mAb-treated xenografts compared with untreated controls (P = 0.087). In addition, we provide evidence that EMMPRIN suppression results in decreased interleukin 1beta (IL-1beta), IL-6, and IL-8 cytokine production, in vitro and in vivo.
Conclusions: These data suggest that anti-EMMPRIN antibody inhibits tumor cell proliferation in vivo and may represent a novel targeted treatment option in head and neck squamous cell carcinoma.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures
Similar articles
-
Modulation of tumor cell growth in vivo by extracellular matrix metalloprotease inducer.Arch Otolaryngol Head Neck Surg. 2008 Nov;134(11):1218-24. doi: 10.1001/archotol.134.11.1218. Arch Otolaryngol Head Neck Surg. 2008. PMID: 19015455 Free PMC article.
-
Anti-EMMPRIN antibody treatment of head and neck squamous cell carcinoma in an ex-vivo model.Anticancer Drugs. 2010 Oct;21(9):861-7. doi: 10.1097/CAD.0b013e32833d1a11. Anticancer Drugs. 2010. PMID: 20700044 Free PMC article.
-
Dual combination therapy targeting DR5 and EMMPRIN in pancreatic adenocarcinoma.Mol Cancer Ther. 2012 Feb;11(2):405-15. doi: 10.1158/1535-7163.MCT-11-0581. Epub 2011 Dec 27. Mol Cancer Ther. 2012. PMID: 22203731 Free PMC article.
-
Dynamic contrast-enhanced MRI evaluates the early response of human head and neck tumor xenografts following anti-EMMPRIN therapy with cisplatin or irradiation.J Magn Reson Imaging. 2015 Oct;42(4):936-45. doi: 10.1002/jmri.24871. Epub 2015 Feb 20. J Magn Reson Imaging. 2015. PMID: 25704985 Free PMC article.
-
Epidermal growth factor receptors as a target for cancer treatment: the emerging role of IMC-C225 in the treatment of lung and head and neck cancers.Semin Oncol. 2002 Feb;29(1 Suppl 4):27-36. doi: 10.1053/sonc.2002.31525. Semin Oncol. 2002. PMID: 11894011 Review.
Cited by
-
An epitope-specific novel anti-EMMPRIN polyclonal antibody inhibits tumor progression.Oncoimmunology. 2015 Aug 28;5(2):e1078056. doi: 10.1080/2162402X.2015.1078056. eCollection 2016 Feb. Oncoimmunology. 2015. PMID: 27057452 Free PMC article.
-
CD147 and AGR2 expression promote cellular proliferation and metastasis of head and neck squamous cell carcinoma.Exp Cell Res. 2012 Aug 15;318(14):1788-98. doi: 10.1016/j.yexcr.2012.04.022. Epub 2012 May 30. Exp Cell Res. 2012. PMID: 22659167 Free PMC article.
-
Local blockage of EMMPRIN impedes pressure ulcers healing in a rat model.Int J Clin Exp Pathol. 2015 Jun 1;8(6):6692-9. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26261551 Free PMC article.
-
A novel extracellular drug conjugate significantly inhibits head and neck squamous cell carcinoma.Oral Oncol. 2013 Oct;49(10):991-7. doi: 10.1016/j.oraloncology.2013.07.006. Epub 2013 Aug 3. Oral Oncol. 2013. PMID: 23920309 Free PMC article.
-
A chimeric antibody targeting CD147 inhibits hepatocellular carcinoma cell motility via FAK-PI3K-Akt-Girdin signaling pathway.Clin Exp Metastasis. 2015 Jan;32(1):39-53. doi: 10.1007/s10585-014-9689-7. Epub 2014 Nov 26. Clin Exp Metastasis. 2015. PMID: 25424030
References
-
- Leon X, Quer M, Orus C, del Prado Venegas M. Can cure be achieved in patients with head and neck carcinomas? The problem of second neoplasm. Expert Rev Anticancer Ther. 2001;1:125–33. - PubMed
-
- Casiglia J, Woo SB. A comprehensive review of oral cancer. Gen Dent. 2001;49:72–82. - PubMed
-
- McCawley LJ, Crawford HC, King LE, Jr, Mudgett J, Matrisian LM. A protective role for matrix metallopro-teinase-3 in squamous cell carcinoma. Cancer Res. 2004;64:6965–72. - PubMed
-
- Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res. 2001;264:169–84. - PubMed
-
- Seljelid R, Jozefowski S, Sveinbjornsson B. Tumor stroma. Anticancer Res. 1999;19:4809–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

