Glucagon acts in a dominant manner to repress insulin-induced mammalian target of rapamycin complex 1 signaling in perfused rat liver
- PMID: 19509187
- PMCID: PMC2724106
- DOI: 10.1152/ajpendo.00042.2009
Glucagon acts in a dominant manner to repress insulin-induced mammalian target of rapamycin complex 1 signaling in perfused rat liver
Abstract
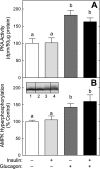
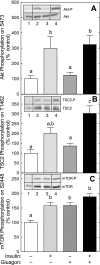
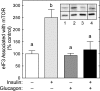
The opposing actions of insulin and glucagon on hepatic carbohydrate metabolism are well documented. In contrast, relatively little is known about how the two hormones interact to regulate hepatic protein metabolism. Previously, we reported that glucagon in the absence of insulin represses signaling through the mammalian target of rapamycin complex 1 (mTORC1). In the present study, we sought to determine whether or not the action of one hormone would dominate over the other in the regulation of mTORC1 signaling. Livers were perfused in situ with medium containing either no added hormones (control), 10 nM insulin, 100 nM glucagon, or a combination of the hormones. Compared with control livers, insulin stimulated Akt phosphorylation and mTORC1 signaling, as assessed by increased phosphorylation of the mTORC1 targets eIF4E-binding protein (4E-BP)1 and ribosomal protein S6 kinase (S6K)1, and promoted assembly of the eIF4G x eIF4E complex. Glucagon alone had no effect on mTORC1 signaling but stimulated the activity of protein kinase A (PKA). In the presence of a combination of insulin and glucagon, Akt and TSC2 phosphorylation and PKA activity were all increased compared with controls. However, mTORC1 signaling was repressed compared with livers perfused with medium containing insulin alone, and this effect was associated with reduced assembly of the mTORC1 x eIF3 complex. Overall, the results suggest that glucagon acts in a dominant manner to repress insulin-induced mTORC1 signaling, which is in contrast to previous studies showing a dominant action of insulin in the control of hepatic gluconeogenesis.
Figures

References
-
- Averous J, Proud CG. When translation meets transformation: the mTOR story. Oncogene 25: 6423–6435, 2006. - PubMed
-
- Baum JI, Layman DK, Freund GG, Rahn KA, Nakamura MT, Yudell BE. A reduced carbohydrate, increased protein diet stabilizes glycemic control and minimizes adipose tissue glucose disposal in rats. J Nutr 136: 1855–1861, 2006. - PubMed
-
- Calbet JA, MacLean DA. Plasma glucagon and insulin responses depend on the rate of appearance of amino acids after ingestion of different protein solutions in humans. J Nutr 132: 2174–2182, 2002. - PubMed
-
- Exton JH The perfused rat liver. Methods Enzymol 39: 25–36, 1975. - PubMed
-
- Feldhoff RC, Taylor JM, Jefferson LS. Synthesis and secretion of rat albumin in vivo, in perfused liver, and in isolated hepatocytes. Effects of hypophysectomy and growth hormone treatment. J Biol Chem 252: 3611–3616, 1977. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

