Harnessing competing endocytic pathways for overcoming the tumor-blood barrier: magnetic resonance imaging and near-infrared imaging of bifunctional contrast media
- PMID: 19509228
- PMCID: PMC2730440
- DOI: 10.1158/0008-5472.CAN-08-4967
Harnessing competing endocytic pathways for overcoming the tumor-blood barrier: magnetic resonance imaging and near-infrared imaging of bifunctional contrast media
Abstract
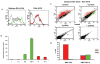
Ovarian cancer is the most lethal gynecologic malignancy, often diagnosed at advanced stage leading to poor prognosis. In the study reported here, magnetic resonance imaging and near-infrared reflectance imaging were applied for in vivo analysis of two competing endocytic pathways affecting retention of bifunctional daidzein-bovine serum albumin (BSA)-based contrast media by human epithelial ovarian carcinoma cells. Suppression of caveolae-mediated uptake using nystatin or by BSA competition significantly enhanced daidzein-BSA-GdDTPA/CyTE777 uptake by tumor cells in vitro. In vivo, perivascular myofibroblasts generated an effective perivascular barrier excluding delivery of BSA-GdDTPA/CyTE777 to tumor cells. The ability to manipulate caveolae-mediated sequestration of albumin by perivascular tumor myofibroblasts allowed us to effectively overcome this tumor-stroma barrier, increasing delivery of daidzein-BSA-GdDTPA/CyTE777 to the tumor cells in tumor xenografts. Thus, both in vitro and in vivo, endocytosis of daidzein-BSA-GdDTPA/CyTE777 by ovarian carcinoma cells was augmented by albumin or by nystatin. In view of the cardinal role of albumin in affecting the availability and pharmacokinetics of drugs, this approach could potentially also facilitate the delivery of therapeutics and contrast media to tumor cells.
Figures


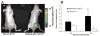


Similar articles
-
Visualizing vascular permeability and lymphatic drainage using labeled serum albumin.Angiogenesis. 2010 Jun;13(2):75-85. doi: 10.1007/s10456-010-9170-4. Epub 2010 May 29. Angiogenesis. 2010. PMID: 20512410 Free PMC article. Review.
-
Labeling fibroblasts with biotin-BSA-GdDTPA-FAM for tracking of tumor-associated stroma by fluorescence and MR imaging.Magn Reson Med. 2005 Oct;54(4):789-97. doi: 10.1002/mrm.20628. Magn Reson Med. 2005. PMID: 16149062 Free PMC article.
-
Preparation and Evaluation of Cabazitaxel-Loaded Bovine Serum Albumin Nanoparticles for Prostate Cancer.Int J Nanomedicine. 2020 Jul 29;15:5333-5344. doi: 10.2147/IJN.S258856. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32801692 Free PMC article.
-
Biotinylated bovine serum albumin linked to gadolinium diethylenetriaminepentaacetic acid.2008 Dec 9 [updated 2008 Dec 26]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Dec 9 [updated 2008 Dec 26]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641367 Free Books & Documents. Review.
-
Conjugates of daidzein-alliinase as a targeted pro-drug enzyme system against ovarian carcinoma.J Drug Target. 2011 Jun;19(5):326-35. doi: 10.3109/1061186X.2010.504265. Epub 2010 Aug 3. J Drug Target. 2011. PMID: 20678009
Cited by
-
Biomimetic material degradation for synergistic enhanced therapy by regulating endogenous energy metabolism imaging under hypothermia.Nat Commun. 2022 Aug 5;13(1):4567. doi: 10.1038/s41467-022-32349-2. Nat Commun. 2022. PMID: 35931744 Free PMC article.
-
Distribution of CPP-Protein Complexes in Freshly Resected Human Tissue Material.Pharmaceuticals (Basel). 2010 Mar 12;3(3):621-635. doi: 10.3390/ph3030621. Pharmaceuticals (Basel). 2010. PMID: 27713271 Free PMC article.
-
Visualizing vascular permeability and lymphatic drainage using labeled serum albumin.Angiogenesis. 2010 Jun;13(2):75-85. doi: 10.1007/s10456-010-9170-4. Epub 2010 May 29. Angiogenesis. 2010. PMID: 20512410 Free PMC article. Review.
References
-
- Schiffenbauer YS, Meir G, Maoz M, Even-Ram SC, Bar-Shavit R, Neeman M. Gonadotropin stimulation of MLS human epithelial ovarian carcinoma cells augments cell adhesion mediated by CD44 and by alpha(v)-integrin. Gynecol Oncol. 2002;84:296–302. - PubMed
-
- Gilead A, Meir G, Neeman M. The role of angiogenesis, vascular maturation, regression and stroma infiltration in dormancy and growth of implanted MLS ovarian carcinoma spheroids. Int J Cancer. 2004;108:524–31. - PubMed
-
- Neeman M, Dafni H, Bukhari O, Braun RD, Dewhirst MW. In vivo BOLD contrast MRI mapping of subcutaneous vascular function and maturation: validation by intravital microscopy. Magn Reson Med. 2001;45:887–98. - PubMed
-
- Gilad AA, Israely T, Dafni H, Meir G, Cohen B, Neeman M. Functional and molecular mapping of uncoupling between vascular permeability and loss of vascular maturation in ovarian carcinoma xenografts: the role of stroma cells in tumor angiogenesis. Int J Cancer. 2005;117:202–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

