GAREM, a novel adaptor protein for growth factor receptor-bound protein 2, contributes to cellular transformation through the activation of extracellular signal-regulated kinase signaling
- PMID: 19509291
- PMCID: PMC2740447
- DOI: 10.1074/jbc.M109.021139
GAREM, a novel adaptor protein for growth factor receptor-bound protein 2, contributes to cellular transformation through the activation of extracellular signal-regulated kinase signaling
Abstract
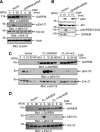
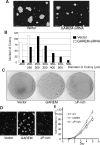
Adaptor proteins for the various growth factor receptors play a crucial role in signal transduction through tyrosine phosphorylation. Several candidates for adaptor proteins with potential effects on the epidermal growth factor (EGF) receptor-mediated signaling pathway have been identified by recent phosphoproteomic studies. Here, we focus on a novel protein, GAREM (Grb2-associated and regulator of Erk/MAPK) as a downstream molecule of the EGF receptor. GAREM is phosphorylated at tyrosine 105 and 453 after EGF stimulation. Grb2 was identified as its binding partner, and the proline-rich motifs of GAREM are recognized by the N- and C-terminal SH3 domains of Grb2. In addition, the tyrosine phosphorylations of GAREM are necessary for its binding to Grb2. Because the amino acid sequence surrounding tyrosine 453 is similar to the immunoreceptor tyrosine-based inhibitory motif, Shp2, a positive regulator of Erk, binds to GAREM in this phosphorylation-dependent manner. Consequently, Erk activation in response to EGF stimulation is regulated by the expression of GAREM in COS-7 and HeLa cells, which occurs independent of the presence of other binding proteins, such as Gab1 and SOS, to the activated EGF receptor. Furthermore, the expression of GAREM has an effect on the transformation activity of cultured cells. Together, these findings suggest that GAREM plays a key role in the ligand-mediated signaling pathway of the EGF receptor and the tumorigenesis of cells.
Figures





Similar articles
-
A brain-specific Grb2-associated regulator of extracellular signal-regulated kinase (Erk)/mitogen-activated protein kinase (MAPK) (GAREM) subtype, GAREM2, contributes to neurite outgrowth of neuroblastoma cells by regulating Erk signaling.J Biol Chem. 2013 Oct 11;288(41):29934-42. doi: 10.1074/jbc.M113.492520. Epub 2013 Sep 3. J Biol Chem. 2013. PMID: 24003223 Free PMC article.
-
Signaling adaptor ShcD suppresses extracellular signal-regulated kinase (Erk) phosphorylation distal to the Ret and Trk neurotrophic receptors.J Biol Chem. 2017 Apr 7;292(14):5748-5759. doi: 10.1074/jbc.M116.770511. Epub 2017 Feb 17. J Biol Chem. 2017. PMID: 28213521 Free PMC article.
-
Tyrosine phosphorylation of Grb2 by Bcr/Abl and epidermal growth factor receptor: a novel regulatory mechanism for tyrosine kinase signaling.EMBO J. 2001 Dec 3;20(23):6793-804. doi: 10.1093/emboj/20.23.6793. EMBO J. 2001. PMID: 11726515 Free PMC article.
-
Regulation of growth factor signaling by FRS2 family docking/scaffold adaptor proteins.Cancer Sci. 2008 Jul;99(7):1319-25. doi: 10.1111/j.1349-7006.2008.00840.x. Epub 2008 Apr 29. Cancer Sci. 2008. PMID: 18452557 Free PMC article. Review.
-
The Configuration of GRB2 in Protein Interaction and Signal Transduction.Biomolecules. 2024 Feb 22;14(3):259. doi: 10.3390/biom14030259. Biomolecules. 2024. PMID: 38540680 Free PMC article. Review.
Cited by
-
Exome sequencing and subsequent association studies identify five amino acid-altering variants influencing human height.Hum Genet. 2012 Mar;131(3):471-8. doi: 10.1007/s00439-011-1096-4. Epub 2011 Sep 29. Hum Genet. 2012. PMID: 21959382
-
A Decade of GWAS Results in Lung Cancer.Cancer Epidemiol Biomarkers Prev. 2018 Apr;27(4):363-379. doi: 10.1158/1055-9965.EPI-16-0794. Epub 2017 Jun 14. Cancer Epidemiol Biomarkers Prev. 2018. PMID: 28615365 Free PMC article. Review.
-
Early signaling dynamics of the epidermal growth factor receptor.Proc Natl Acad Sci U S A. 2016 Mar 15;113(11):3114-9. doi: 10.1073/pnas.1521288113. Epub 2016 Feb 29. Proc Natl Acad Sci U S A. 2016. PMID: 26929352 Free PMC article.
-
Integrated analysis of lncRNAs and mRNAs by RNA-Seq in secondary hair follicle development and cycling (anagen, catagen and telogen) of Jiangnan cashmere goat (Capra hircus).BMC Vet Res. 2022 May 6;18(1):167. doi: 10.1186/s12917-022-03253-0. BMC Vet Res. 2022. PMID: 35524260 Free PMC article.
-
MCAM: multiple clustering analysis methodology for deriving hypotheses and insights from high-throughput proteomic datasets.PLoS Comput Biol. 2011 Jul;7(7):e1002119. doi: 10.1371/journal.pcbi.1002119. Epub 2011 Jul 21. PLoS Comput Biol. 2011. PMID: 21799663 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

