Requirement of histone methyltransferase SMYD3 for estrogen receptor-mediated transcription
- PMID: 19509295
- PMCID: PMC2740412
- DOI: 10.1074/jbc.M109.021485
Requirement of histone methyltransferase SMYD3 for estrogen receptor-mediated transcription
Abstract
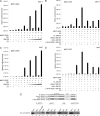
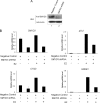
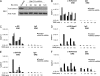
SMYD3 is a SET domain-containing protein with histone methyltransferase activity on histone H3-K4. Recent studies showed that SMYD3 is frequently overexpressed in different types of cancer cells, but how SMYD3 regulates the development and progression of these malignancies remains unknown. Here, we report the previously unrecognized role of SMYD3 in estrogen receptor (ER)-mediated transcription via its histone methyltransferase activity. We demonstrate that SMYD3 functions as a coactivator of ERalpha and potentiates ERalpha activity in response to ligand. SMYD3 directly interacts with the ligand binding domain of ER and is recruited to the proximal promoter regions of ER target genes upon gene induction. Importantly, our chromatin immunoprecipitation analyses provide compelling evidence that SMYD3 is responsible for the accumulation of di- and trimethylation of H3-K4 at the induced ER target genes. Furthermore, RNA interference-directed down-regulation of SMYD3 reveals that SMYD3 is required for ER-regulated gene transcription in estrogen signaling pathway. Thus, our results identify SMYD3 as a new coactivator for ER-mediated transcription, providing a possible link between SMYD3 overexpression and breast cancer.
Figures




Similar articles
-
The telomerase reverse transcriptase (hTERT) gene is a direct target of the histone methyltransferase SMYD3.Cancer Res. 2007 Mar 15;67(6):2626-31. doi: 10.1158/0008-5472.CAN-06-4126. Cancer Res. 2007. PMID: 17363582
-
SMYD3 encodes a histone methyltransferase involved in the proliferation of cancer cells.Nat Cell Biol. 2004 Aug;6(8):731-40. doi: 10.1038/ncb1151. Epub 2004 Jul 4. Nat Cell Biol. 2004. PMID: 15235609
-
Transcriptional regulation of 15-lipoxygenase expression by histone h3 lysine 4 methylation/demethylation.PLoS One. 2012;7(12):e52703. doi: 10.1371/journal.pone.0052703. Epub 2012 Dec 28. PLoS One. 2012. PMID: 23285160 Free PMC article.
-
SMYD3: a regulator of epigenetic and signaling pathways in cancer.Clin Epigenetics. 2021 Feb 26;13(1):45. doi: 10.1186/s13148-021-01021-9. Clin Epigenetics. 2021. PMID: 33637115 Free PMC article. Review.
-
Lysine methylation in cancer: SMYD3-MAP3K2 teaches us new lessons in the Ras-ERK pathway.Bioessays. 2014 Dec;36(12):1162-9. doi: 10.1002/bies.201400120. Epub 2014 Sep 2. Bioessays. 2014. PMID: 25382779 Review.
Cited by
-
The methyltransferase SMYD3 mediates the recruitment of transcriptional cofactors at the myostatin and c-Met genes and regulates skeletal muscle atrophy.Genes Dev. 2013 Jun 1;27(11):1299-312. doi: 10.1101/gad.217240.113. Genes Dev. 2013. PMID: 23752591 Free PMC article.
-
Playing on the Dark Side: SMYD3 Acts as a Cancer Genome Keeper in Gastrointestinal Malignancies.Cancers (Basel). 2021 Sep 2;13(17):4427. doi: 10.3390/cancers13174427. Cancers (Basel). 2021. PMID: 34503239 Free PMC article. Review.
-
Epigenetic modifications as therapeutic targets.Nat Biotechnol. 2010 Oct;28(10):1069-78. doi: 10.1038/nbt.1678. Nat Biotechnol. 2010. PMID: 20944599 Free PMC article. Review.
-
The transcription factor HOXA9 induces expression of the chromatin modifier SMYD3 to drive leukemogenesis.J Biol Chem. 2025 Jul;301(7):110320. doi: 10.1016/j.jbc.2025.110320. Epub 2025 May 30. J Biol Chem. 2025. PMID: 40451434 Free PMC article.
-
In Silico/In Vitro Hit-to-Lead Methodology Yields SMYD3 Inhibitor That Eliminates Unrestrained Proliferation of Breast Carcinoma Cells.Int J Mol Sci. 2020 Dec 15;21(24):9549. doi: 10.3390/ijms21249549. Int J Mol Sci. 2020. PMID: 33333978 Free PMC article.
References
-
- McKenna N. J., Lanz R. B., O'Malley B. W. (1999) Endocr. Rev. 20, 321–344 - PubMed
-
- Métivier R., Penot G., Hübner M. R., Reid G., Brand H., Kos M., Gannon F. (2003) Cell 115, 751–763 - PubMed
-
- Tsai M. J., O'Malley B. W. (1994) Annu. Rev. Biochem. 63, 451–486 - PubMed
-
- Enmark E., Gustafsson J. A. (1999) J. Intern. Med. 246, 133–138 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

