Intranasal deferoxamine provides increased brain exposure and significant protection in rat ischemic stroke
- PMID: 19509317
- PMCID: PMC2729791
- DOI: 10.1124/jpet.108.149807
Intranasal deferoxamine provides increased brain exposure and significant protection in rat ischemic stroke
Abstract
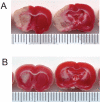
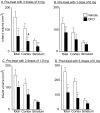
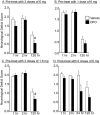
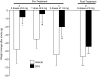
Deferoxamine (DFO) is a high-affinity iron chelator approved by the Food and Drug Administration for treating iron overload. Preclinical research suggests that systemically administered DFO prevents and treats ischemic stroke damage and intracerebral hemorrhage. However, translation into human trials has been limited, probably because of difficulties with DFO administration. A noninvasive method of intranasal administration has emerged recently as a rapid way to bypass the blood-brain barrier and target therapeutic agents to the central nervous system. We report here that intranasal administration targets DFO to the brain and reduces systemic exposure, and that intranasal DFO prevents and treats stroke damage after middle cerebral artery occlusion (MCAO) in rats. A 6-mg dose of DFO resulted in significantly higher DFO concentrations in the brain (0.9-18.5 microM) at 30 min after intranasal administration than after intravenous administration (0.1-0.5 microM, p < 0.05). Relative to blood concentration, intranasal delivery increased targeting of DFO to the cortex approximately 200-fold compared with intravenous delivery. Intranasal administration of three 6-mg doses of DFO did not result in clinically significant changes in blood pressure or heart rate. Pretreatment with intranasal DFO (three 6-mg doses) 48 h before MCAO significantly decreased infarct volume by 55% versus control (p < 0.05). In addition, post-treatment with intranasal administration of DFO (six 6-mg doses) immediately after reperfusion significantly decreased infarct volume by 55% (p < 0.05). These experiments suggest that intranasally administered DFO may be a useful treatment for stroke, and a prophylactic for patients at high risk for stroke.
Figures
References
-
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, and Bartkowski H (1986) Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 17 472-476. - PubMed
-
- Belayev L, Alonso OF, Busto R, Zhao W, and Ginsberg MD (1996) Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke 27 1616-1623. - PubMed
-
- Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, and Kern W (2004) Intranasal insulin improves memory in humans. Psychoneuroendocrinology 29 1326-1334. - PubMed
-
- Benedict C, Hallschmid M, Schmitz K, Schultes B, Ratter F, Fehm HL, Born J, and Kern W (2007) Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology 32 239-243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

