Pentacyclic triterpene distribution in various plants - rich sources for a new group of multi-potent plant extracts
- PMID: 19513002
- PMCID: PMC6254168
- DOI: 10.3390/molecules14062016
Pentacyclic triterpene distribution in various plants - rich sources for a new group of multi-potent plant extracts
Abstract
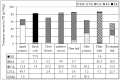
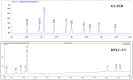
Pentacyclic triterpenes are secondary plant metabolites widespread in fruit peel, leaves and stem bark. In particular the lupane-, oleanane-, and ursane triterpenes display various pharmacological effects while being devoid of prominent toxicity. Therefore, these triterpenes are promising leading compounds for the development of new multi-targeting bioactive agents. Screening of 39 plant materials identified triterpene rich (> 0.1% dry matter) plant parts. Plant materials with high triterpene concentrations were then used to obtain dry extracts by accelerated solvent extraction resulting in a triterpene content of 50 - 90%. Depending on the plant material, betulin (birch bark), betulinic acid (plane bark), oleanolic acid (olive leaves, olive pomace, mistletoe sprouts, clove flowers), ursolic acid (apple pomace) or an equal mixture of the three triterpene acids (rosemary leaves) are the main components of these dry extracts. They are quantitatively characterised plant extracts supplying a high concentration of actives and therefore can be used for development of phytopharmaceutical formulations.
Figures



References
-
- Juan M.E., Wenzel U., Ruiz-Gutierrez V., Daniel H., Planas J.M. Olive fruit extracts inhibit proliferation and induce apoptosis in HT-29 human colon cancer cells. J. Nutr. 2006;136:2553–2557. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

