Aging and replicative senescence have related effects on human stem and progenitor cells
- PMID: 19513108
- PMCID: PMC2688074
- DOI: 10.1371/journal.pone.0005846
Aging and replicative senescence have related effects on human stem and progenitor cells
Abstract
The regenerative potential diminishes with age and this has been ascribed to functional impairments of adult stem cells. Cells in culture undergo senescence after a certain number of cell divisions whereby the cells enlarge and finally stop proliferation. This observation of replicative senescence has been extrapolated to somatic stem cells in vivo and might reflect the aging process of the whole organism. In this study we have analyzed the effect of aging on gene expression profiles of human mesenchymal stromal cells (MSC) and human hematopoietic progenitor cells (HPC). MSC were isolated from bone marrow of donors between 21 and 92 years old. 67 genes were age-induced and 60 were age-repressed. HPC were isolated from cord blood or from mobilized peripheral blood of donors between 27 and 73 years and 432 genes were age-induced and 495 were age-repressed. The overlap of age-associated differential gene expression in HPC and MSC was moderate. However, it was striking that several age-related gene expression changes in both MSC and HPC were also differentially expressed upon replicative senescence of MSC in vitro. Especially genes involved in genomic integrity and regulation of transcription were age-repressed. Although telomerase activity and telomere length varied in HPC particularly from older donors, an age-dependent decline was not significant arguing against telomere exhaustion as being causal for the aging phenotype. These studies have demonstrated that aging causes gene expression changes in human MSC and HPC that vary between the two different cell types. Changes upon aging of MSC and HPC are related to those of replicative senescence of MSC in vitro and this indicates that our stem and progenitor cells undergo a similar process also in vivo.
Conflict of interest statement
Figures

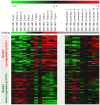



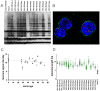
References
-
- Gazit R, Weissman IL, Rossi DJ. Hematopoietic stem cells and the aging hematopoietic system. Semin Hematol. 2008;45:218–224. - PubMed
-
- Horwitz EM, Le BK, Dominici M, Mueller I, Slaper-Cortenbach I, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. - PubMed
-
- Horn P, Bork S, Diehlmann A, Walenda T, Eckstein V, et al. Isolation of human mesenchymal stromal cells is more efficient by red blood cell lysis. Cytotherapy. 2008;10:676–685. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

