SHMT1 and SHMT2 are functionally redundant in nuclear de novo thymidylate biosynthesis
- PMID: 19513116
- PMCID: PMC2688753
- DOI: 10.1371/journal.pone.0005839
SHMT1 and SHMT2 are functionally redundant in nuclear de novo thymidylate biosynthesis
Abstract
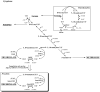
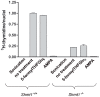
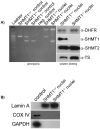
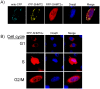
The three enzymes that constitute the de novo thymidylate synthesis pathway in mammals, cytoplasmic serine hydroxymethyltransferase (SHMT1), thymidylate synthase (TYMS) and dihydrofolate reductase (DHFR) undergo sumoylation and nuclear import during S-phase. In this study, we demonstrate that purified intact mouse liver nuclei convert dUMP to dTMP in the presence of NADPH and serine. Neither nuclear extracts nor intact nuclei exposed to aminomethylphosphonate, a SHMT inhibitor, exhibit thymidylate synthesis activity. Nuclei isolated from Shmt1(-/-) mouse livers retained 25% of thymidylate synthesis activity exhibited by nuclei isolated from wild type mice. This residual activity was due to the presence of a cytoplasmic/nuclear isozyme of SHMT encoded by Shmt2. Shmt2 is shown to encode two transcripts, one which encodes a protein that localizes exclusively to the mitochondria (SHMT2), and a second transcript that lacks exon 1 and encodes a protein that localizes to the cytoplasm and nucleus during S-phase (SHMT2alpha). The ability of Shmt2 to encode a cytoplasmic isozyme of SHMT may account for the viability of Shmt1(-/-) mice and provide redundancy that permitted the expansion of the human SHMT1 L474F polymorphism that impairs SHMT1 sumoylation and nuclear translocation.
Conflict of interest statement
Figures



References
-
- Fox JT, Stover PJ. Folate-mediated one-carbon metabolism. Vitam Horm. 2008;79:1–44. - PubMed
-
- Garrow TA, Brenner AA, Whitehead VM, Chen XN, Duncan RG, et al. Cloning of human cDNAs encoding mitochondrial and cytosolic serine hydroxymethyltransferases and chromosomal localization. J Biol Chem. 1993;268:11910–11916. - PubMed
-
- Girgis S, Nasrallah IM, Suh JR, Oppenheim E, Zanetti KA, et al. Molecular cloning, characterization and alternative splicing of the human cytoplasmic serine hydroxymethyltransferase gene. Gene. 1998;210:315–324. - PubMed
-
- Stover PJ, Chen LH, Suh JR, Stover DM, Keyomarsi K, et al. Molecular cloning, characterization, and regulation of the human mitochondrial serine hydroxymethyltransferase gene. J Biol Chem. 1997;272:1842–1848. - PubMed
-
- Appling DR. Compartmentation of folate-mediated one-carbon metabolism in eukaryotes. Faseb J. 1991;5:2645–2651. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

